One of the major strengths of JILA are the frequent and ongoing collaborations between experimentalists and theorists, which have led to incredible discoveries in physics. One of these partnerships is between JILA Fellow John Bohn and JILA and NIST Fellow Jun Ye. Bohn's team of theorists has partnered with Ye's experimentalist laboratory for nearly twenty years, from the very beginning of Ye’s cold molecule research when he became a JILA Fellow. Recently in their collaborations, the researchers have been studying a three-dimensional molecular gas made of 40K87Rb molecules. In a paper published in Nature Physics, the combined team illustrated new quantum mechanical tricks in making this gas unreactive, thus enjoying a long life (for a gas), while at the same time letting the molecules in the gas interact and socialize (thermalize) with each other.
Creating a Gas
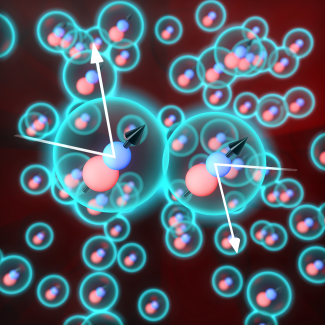
The work of creating this ultracold three-dimensional gas at JILA began more than 10 years ago with Fellows Jun Ye and Deborah Jin. Once they had created the gas, it kept reacting. In fact, many research groups worldwide have now achieved similar types of molecular gases in the quantum regime, and everybody has had to deal with the loss of molecules due to chemical reaction. Bohn and his team worked with Ye and his team to come up with a theory for stopping this reaction. The postulated theory involved a process called resonant shielding. “The shielding says, basically, if you take these molecules, and you put them in their quantum mechanical ground state, they attract each other, like all atoms or molecules attract each other by so called Van der Waals forces,” Bohn explained. “That's a quantum mechanical thing that goes way, way back. And that's what we're trying to avoid. Because if the molecules attract each other, they run into each other and react chemically, changing themselves.” In order to avoid chemical reactions, the molecular gas goes though the resonant shielding process where: “If you put the molecule in an excited state where it's already rotating, then you put the electric field just so, then the fluctuations in this dipole orientation make the molecules repel each other,” Bohn added. Because of the repulsion, the molecules in the gas don't get close enough with each other to react, thereby allowing for better control of the gas, such as turning on the dipolar interactions that remain effective at longer distances. From the data in the recently published paper, Bohn explained what the team found when the data was plotted: “The rate at which chemical reactions are happening, and it's high, high, high, and the experiment gets to a certain field–then bam! It drops by two orders of magnitude.” The fine-tuning of this gas allowed the researchers to examine other aspects of the gas without worrying about causing chemical reactions. Bohn's team theorized the resonant shielding as a recipe for creating this inert gas. Ye’s team then took the theory and began experimenting in the laboratory.
Cooling It Down
The opportunity of studying the unreactive gas was too good for the researchers to pass up. According to first author Jun-Ru Li: “Achieving such a collision resonance allows us to do the evaporative cooling of the molecules, which produces molecular gases with very low entropy. This allows us to use this platform to study a range of many-body phenomena related to dipolar quantum gases.” The many-body problem has intrigued physicists for years, as it refers to a category of issues relating to microscopic systems with interacting particles. Having an ultra-cold three-dimensional molecular gas that didn't react is valuable for the researchers to engineer and tune interparticle interactions that can give rise to exotic quantum many-body dynamics.
The researchers looked into the evaporative cooling of the gas. The process of evaporative cooling is what cools your body when you sweat. As the sweat evaporates, its molecules use heat energy to convert from a liquid to a vapor and escape from the surface of your skin, leading to a decrease in temperature as the heat is transferred into the evaporating sweat. In order to measure the evaporative cooling of their inert gas, the team looked at the gas’s thermalization signature which contributed to evaporative cooling. “It's the number of collisions between particles that is required for them to thermalize with each other,” graduate student Reuben Wang said. “We measure this signature to determine if the gas is interacting while not reacting. And it very nicely tallies with our theoretical prediction.” The researchers were excited to see the theory and experiment corroborate each other. “Those data are explained beautifully by Reuben's theories,” Bohn stated. “This is the first ultracold molecular gas in three dimensions where they saw evaporative cooling.” Bohn and the rest of the team looked forward to testing other characteristics of this gas.
A Valuable Collaboration
In looking back on the research, Bohn was grateful to have the partnership with Ye's laboratory. “That is one of the strengths of JILA as a whole, is a strong experimental-theory coupling. Not only is it a lot of fun, but it makes the science go faster.” Bohn has been at JILA long enough to understand the dynamics of this relationship. “Any number of times, an experimentalist will see something. And they burst into the theorist's office and say: ‘What is this?’” As a collaborating experimentalist, Li is thankful for the effort done by the theorists. “We achieve something in the lab, and then the theorists begin working on that. And then the theory predicts something even more interesting. And we use experiment to explore the theory. So, it's kind of a back-and-forth process of helping each other.” As the collaboration continues, the research becomes more rewarding, as both types of physicists share in their hard work to advance knowledge and develop new technology.
Written by Kenna Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.