Light is emitted when an atom decays from an excited state to a lower energy ground state, with the emitted photon carrying away the energy. The spontaneous emission of light is a fundamental process that originates from the interaction between matter and the modes of the electromagnetic field—the background “hiss” of the universe that is all around us. However, spontaneous emission of light can limit the utility of atomic excited states for a wide array of scientific and technological applications, from probing the nature of the universe to inertial navigation. Understanding ways to alter or even engineer spontaneous emission has been an intriguing topic in science. JILA Fellows Ana Maria Rey and James Thompson study ways to control light emission by placing atoms in an optical cavity, a resonator made of two mirrors between which light can bounce back and forth many times. Together, with JILA postdoc and first author Asier Piñeiro Orioli, they have predicted that when an array of multi-level atoms is placed in the cavity the atoms can all cooperate and collectively suppress their emission of light into the cavity. These findings were recently published in Physical Review X.
The Paths of Emission
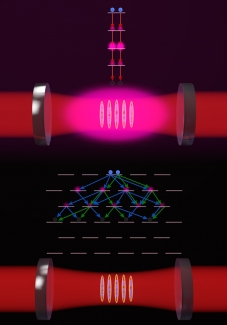
As an atom goes through spontaneous emission, it follows a certain decay path, i.e., the different energy levels an atom passes through on the process of decaying. This process can be modified if many atoms are placed inside a cavity where atoms become highly sensitive to the light emitted by the other atoms. For two-level atoms—atoms with only two internal levels: a ground and an excited state—the decay path is rather straightforward. When an atom decays from the excited state to the ground state it stimulates other atoms to do the same, i.e., the decay paths interfere constructively. As a consequence, all atoms collectively emit light at a faster rate; a process known as superradiance, a phenomenon that has been observed in experiments performed by the Thompson Group. However, for multi-level atoms, things can get a bit complicated. “What happens is that when an excited multilevel atom starts to radiate there are multiple paths it can go through,” explained Rey. This is different from a two-level atom which has only one path. “Interestingly, there are many situations where the various paths interfere destructively, causing a cancellation in the decay process,” Rey added. “When that happens, atoms get stuck in a particular state and stop emitting light into the cavity.” This state is called a quantum dark state, or, in contrast to superradiance, a subradiant state. As Thompson describes, “It is kind of like a corn maze with many paths from entrance to exit. If you go down a single path, you will always reach the exit. But in quantum mechanics, the atoms will try to follow each path through the maze simultaneously, and they can cancel each other’s ability to ever exit the maze—super weird, right?”
One way to visualize this quantum corn maze is by imagining an atom as an arrow that points down when it is in the ground state and up when it is in the excited state. In this scenario, the light emission process can be seen as a rotation of the arrow from up to down, where the rotation speed depends on the sum of all the arrows. When you have two-level atoms in the cavity, all arrows synchronize in rotation resulting in a very big “arrow”. However, multilevel atoms are different, according to Piñeiro Orioli: “Imagine we can visualize each multilevel atom, not as a single arrow, but instead as a collection of arrows which are associated with the different decay transitions available. During the emission process the arrows start to rotate back down towards the ground state but this time you can encounter a situation where the arrows point in different directions such that their sum kind of cancels out. When this happens, the arrows stop rotating and the atoms get stuck. This is what we call a quantum dark state.”
A quantum dark state may offer many benefits for quantum technology, including quantum clocks, which many JILAns study. Not only is the quantum dark state intriguing, but the researchers found that the state itself was inherently entangled. As Rey noted, “To be dark, these states must have some correlations. These particles need to know what other particles are doing to avoid the decay process.” Thompson adds, “By using many internal levels, the extra paths allow for correlations that could even move beyond classical correlations and involve quantum entanglement between the atoms.” The entangled properties of the dark states make them even more attractive for future quantum technologies.
Preparing Dark States
In order to observe these dark states, the team of researchers proposed an experiment that utilizes strontium atoms in an optical cavity, an experimental setup that exists in the Thompson laboratory. Strontium atoms, Rey explained, have: “a narrow transition with 10 excited levels and a spontaneous emission decay rate that can be longer than 100 seconds. This allows the strontium atoms to have multiple decay paths, permitting them to give rise to quantum dark states. According to Rey: “To create these dark states, we would first drive the cavity, which means we inject light in the cavity with certain polarization. We add coherent light into the cavity for some time. Then we stop the driving process and let the atoms evolve until they all decay to the ground stay or get stuck in a quantum dark state.”
For realistic implementations of their proposed experiment, the team of researchers provides a more nuanced view on the power of their findings. “The dark states we have found are not perfect,” Piñeiro Orioli said. “They are only dark when they decay into the cavity, which is by far the dominant decay channel. However, they will still decay through spontaneous emission into free space, i.e., photons will be emitted into other directions. But, if we choose a narrow transition, this free space decay will be very slow and we will be able to see the benefit from the cavity's dark nature of the state.”
Future Dark States
Moving forward, the researchers are hoping to further improve their quantum dark states. “In the future, we want to figure out ways to create large scale entangled states while retaining the darkness in the cavity decay,” added Piñeiro Orioli. “I believe this is possible because the large number of dark states available gives us the freedom to combine them in many different ways. Some preliminary results make me optimistic, but this is still a work in progress and we will have to wait.”
Written by Kenna Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.