In 2008, the Ye and Jin groups succeeded in making ultracold potassium-rubidium (KRb) molecules in their ground state (See “Redefining Chemistry at JILA” in the Spring 2010 issue of JILA Light & Matter). Their next goal was to figure out how to precisely control chemical reactions of these ultracold polar molecules by manipulating the quantum states of the reactants. But first the researchers had to discover how to calm those reactions down enough to study them. Under the conditions in which they were made (an optical trap allowing motion in all three dimensions), ultracold KRb molecules were so chemically reactive they disappeared almost as soon as they were formed.
Then, with help from their theorist colleagues in the Bohn group, the researchers found out that when an electric field is present, the KRb molecules would get even more reactive. In an electric field, the fast chemical reactions started looking like explosions. Explosions are pretty cool for chemists. But they’re a nightmare for experimental physicists, especially if their goal is to cool a gas of polar KRb molecules down to the temperature where all their quantum states occupy the lowest possible energy levels. This state of affairs is called quantum degeneracy. To get there, the KRb molecules have to collide, but not react.
Fortunately, research associate Goulven Quéméner and Fellow John L. Bohn developed a theory for seriously suppressing the reaction rate of KRb molecules. Then, a team from the Ye and Jin groups proved the method worked! The experimental team was led by Marcio de Miranda (now at the Universidade de São Paulo), research associate Amodsen Chotia, graduate student Brian Neyenhuis, and former research associate Dajun Wang (now at the Chinese University of Hong Kong). The team also included former research associate Silke Ospelkaus (now at the University of Hanover in Germany) as well as Fellows Jun Ye and Debbie Jin.
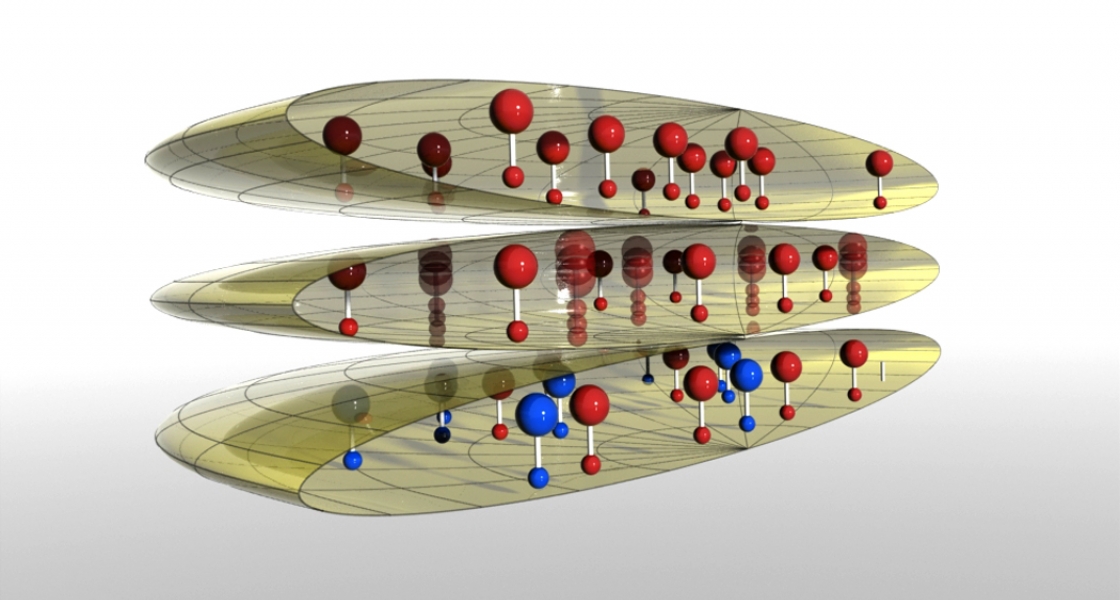
Here’s what the experimental team did to slow down the chemical reactions: First, the researchers squeezed ultracold KRb molecules into a two-dimensional pancake trap. This trap forced the molecules to line up side by side with identical ends of the molecules next to each other. This step mostly prevents the molecules from aligning head-to-tail, which enhances chemical reactions in a major way because opposite ends of dipoles attract one another. Second, the experimentalists made sure the squeezed molecules were in the same quantum state. This step is really important because KRb molecules are fermions. Fermions in the same state mostly bounce off each other rather than reacting. However, fermions that are in slightly different states can collide and react. For example, if otherwise identical fermions are rotating at different rates, they can react inside a pancake trap. Third, the Ye/Jin team turned on an electric field that increased the repulsion between the side-to-side molecules.
The new method increases the lifetime of the KRb molecules to one second. It also makes it nearly a hundred times more likely that KRb molecules will bounce off one another rather than chemically react. This important result (reported in Nature Physics) means that the experimental team should be able to cool their gas of dipolar KRb molecules with evaporative cooling. In evaporative cooling, repeated collisions that don’t change the quantum states of the molecules will result in the gas getting colder and colder — ideally, until the gas reaches quantum degeneracy. Making a quantum degenerate gas of KRb molecules will open the door to exploring the quantum nature of dipolar molecules and their reactivity over long distances. It may even one day lead to the creation of states of matter never before seen in a laboratory! - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.