Worldwide, many researchers are interested in controlling atomic and molecular interactions. This includes JILA and NIST fellows Jun Ye and Ana Maria Rey, both of whom have spent years researching interacting potassium-rubidium (KRb) molecules, which were originally created in a collaboration between Ye and the late Deborah Jin. In the newest collaboration between the experimental (Ye) and theory (Rey) groups, the researchers have developed a new way to control two-dimensional gaseous layers of molecules, publishing their exciting new results in the journal Science.
Electricity as a Tuning Knob
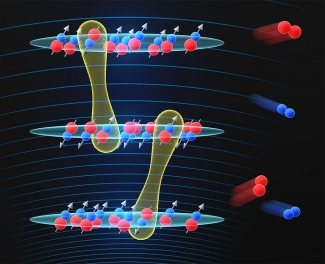
Building off their previous work, the researchers created ultracold KRb molecules in a stack of two-dimensional layers. This time, they applied an electric field gradient to manipulate the energies of the molecules and the interactions between layers. First author and Ye group graduate student William Tobias explained the setup of the experiment: “It's a one-dimensional optical lattice, which arranges molecules like a stack of pancakes. The molecules can each move in a two-dimensional plane, but not between layers.” Because the molecules within these layers are polar (having positive and negative poles), they can be influenced by electric fields which act on the charges that these poles have. “Polar molecules are particularly interesting because they have dipolar interactions, which are typically longer ranged than interactions between atoms and are anisotropic, which means that the interaction varies based on the relative orientation of the molecules,” he added. Dipolar interactions occur when the charged poles on these molecules interact, causing them to attract or repel each other.
Using these charged poles, the team manipulated the dipolar interactions via an electric field. “Our new method uses electric fields to select individual layers of molecules and prepare them in specific rotational states,” Tobias stated. Molecules in different rotational states, which are energy levels determined by the speed of rotation, respond differently to electric fields. “Starting from an initial condition of many layers containing molecules, we can reduce the system to only one or two layers by probing the molecules with microwaves and prepare quantum systems with controlled densities and interactions.” This extra level of electric field control worked like a fine-tuning knob, where the researchers could probe each layer individually and study the molecular interactions more closely.
A New Molecule-Scale Microscope
The researchers were even able to use their molecules as an electric field microscope by manipulating the energy of the molecules using the electric field. “If you stabilize the molecules very carefully with respect to the electric field”, Tobias said, “you can either apply a known electric field to control the molecules or use the molecules to measure an unknown electric field distribution. We showed that we could measure electric fields with a spatial precision on the order of tens of nanometers.” This was especially important when the researchers were studying a molecular phenomenon called “spin exchange.”
Spinning into Different Energy States
When looking at molecular interactions, rotation is important—interacting molecules can trade their rotational states, swapping energy levels in a process called “spin exchange.” This can even happen between the different layers within the researchers' experiments. “Once you're able to select the individual layers of the lattice, if you prepare molecules in two layers in different rotational states, you find that they exchange rotational states between layers,” Tobias noted. This exchange affects the energy levels of these molecules. “Exchanging states conserves energy if there is no difference in the electric field between layers,” Tobias added. “But if we introduce an electric field difference [a variation in field between neighboring layers], it costs some energy for the molecules to make that exchange. We found in the experiment that when the molecules trade rotational states, they also have to give up or gain kinetic energy as well, equal to the change in the electric potential energy.” The Rey group, for their part of the collaboration, worked to help calculate the dependence of the spin exchange rate on the electric field difference. From their calculations and the experiments from the Ye group, the researchers found that a larger electric field difference minimized the spin-exchange rate, allowing for better control of the system.
With their “fine-tuning knobs”, the researchers could better understand and control the molecular interactions within the compressed layers. This new precision allowed the team to develop other aspects of these interactions to study. According to Tobias: “One project we're working on right now is to measure energy shifts from thousands of polar molecules in single and multiple layers using the tools we've developed for layer selection. In the longer term, although we would need to be at much lower temperatures, there are a variety of proposals for using pairing of molecules between layers to study superfluidity and other quantum phenomena.” The researchers are hopeful other physicists will use their methods to further explore molecular interactions.
Written by Kenna Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.