Quantum gases of interacting molecules can exhibit unique dynamics. JILA and NIST Physicist Jun Ye has spent years of research to reveal, probe, and control these dynamics with potassium-rubidium molecules. In a new article published in Nature, Ye and his team of researchers describe having combined two threads of previous research—spin and motional dynamics—to reveal rich many-body and collisional physics that are controllable in the laboratory.
A History Lesson in Quantum Dynamics
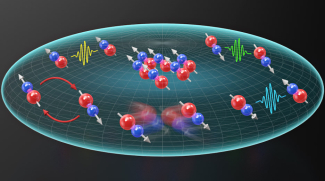
The rotation of polar molecules can be used to encode or “program” the spin of particles, like electrons. Previous research has shown that the rotational states of molecules can be controlled using microwave pulses, allowing physicists to use molecules to study the dynamics of spin systems. Building on fruitful collaboration between former JILA Fellow Deborah Jin and Ye, together with the theory group of Ana Maria Rey, the research team has been looking at spin dynamics for about a decade. “The group was synthesizing ultra-cold potassium-rubidium molecules in three-dimensional optical interference patterns” explained postdoctoral researcher Jacob ‘Jake’ Higgins. “You can think of them as a three-dimensional checkerboard, and each molecule fits into one of the checkerboard sites. The molecules were stuck in zero-dimensional sites, and the group was observing these ‘spin exchanges’ where they can exchange rotational energy.” Molecular interactions like spin exchange can affect molecular collisions and even chemical reactions. Understanding more about these dynamics could help researchers better understand and control quantum systems. The research then moved from a three-dimensional lattice where the molecules are frozen in place to two-dimensional planes or ‘pancakes’ through which the molecules could freely move. In recent years, they have used these pancakes, along with other instrumental capabilities such as tunable electric fields, to control the molecular collisions.
In the latest paper, the Ye group combined the molecular rotation and motion and studied the dynamics arising from this interplay. As Higgins said, “The title of our paper is ‘Tunable Itinerant Spin Dynamics [with Polar Molecules].’ So, it's itinerant, which means the molecules can move. And it’s about spin dynamics, like the spin exchange that the group observed 10 years ago.” With their new setup, the team could further study the influence of motion on the spin dynamics of the system. Furthermor e, the new experimental platform integrates precise control of electric fields and microwave pulses to control spin orientation and coherence which enabled the group to realize a highly tunable, strongly interacting many-body system.
Fighting with the Noise
Even in the ultracold regime, to study quantum dynamics, scientists must find ways to mitigate noise in the system. There are fluctuations in external fields and inhomogeneous, thermal distributions in molecular energies—both of which can obscure subtle signals that arise from the coupled spin-motional dynamics. To mitigate the effects of environmental noise while letting the molecules move, the team instead used microwave pulses in ultracold conditions. As lead author and postdoctoral researcher Jun-Ru Li explained: “There is a very important technique we implemented for this work called dynamical decoupling. Basically, we used microwave pulses to repeatedly change the state of the system such that effects of the environmental noise cancel. Eventually, you can suppress the noise from the environment on average.” Using the microwave pulses, the researchers were able to reduce the effect of the noise by a factor of 70. With a quieter system, the team was able to see a clear signal of shift in the rotational energy resulting from interactions between molecules.
Entering the Quantum Classroom
The collective interaction between molecules shifts their rotational energy levels. With the noise now suppressed, “…we can see a shift in the rotational energy levels of the molecules,” explained graduate student Calder Miller. “This is because the molecules’ electric dipoles generate electric fields, so each molecule ‘senses’ the electric fields that all the other molecules are generating. That [other] electric field shifts the rotational energy of the molecule, and that's something we can measure spectroscopically.” Because the atoms were not frozen, as in a three-dimensional lattice, they could move freely and, in turn, affect nearly all their fellow molecules. “It's a bit like if you are in a classroom where everyone is sitting at different desks, and you want to talk to each other,” added Li. “There are some people sitting far from you and some people sitting close to you. You will hear the words of the people that are close to you much better than the people that are farther away. But now, if you imagine everyone is randomly moving in this classroom, you get the chance to talk to everyone, and a collective exchange might occur.”
The researchers quantified this shift, the mean field shift, and demonstrated its tunability with experimental parameters. “We change the electric field as well as the internal state of the molecules to show that we can control the strength of their interactions, that we can make the interactions attractive or repulsive, or even flip their sign in the middle of the experiment to make the dynamics evolve backward,” Higgins said. With these tunable spin dynamics, the researchers demonstrated how control of the interactions might enable exciting applications. “That's the spirit of what the experiment is. We are really trying to delve into what all these interactions are and how we can tune them. Ultimately, [we want to know] how we can use them for quantum computation and other applications.”
The Unique Setup of the Quantum Classroom
Thanks to this unique experimental setup, the researchers can measure more than the mean field shift of the molecules. “We also see that the system decoheres over time,” Miller added. “This means that the phase of the molecules is randomized. We have some evidence that this is because of elastic collisions between the molecules, which are enabled by the same dipole-dipole interaction that causes the energy shift.”
The creation of this experimental setup also suggests some big implications for other fields of physics. “Ultracold molecules have long been of interest to the scientific community for their potential applications to a variety of fields including quantum chemistry, quantum magnetism, and precision measurement for fundamental physics,” said Annette “Annie” Carroll, a graduate student in Ye's laboratory. “This work paves the way for future applications of polar molecules to study open questions in other areas of condensed matter physics and materials science. Our system could really deepen our understanding of fundamental science in these areas of physics and chemistry.”
Written by Kenna Hughes-Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.