While it may not look like it, the interstellar space between stars is far from empty. Atoms, ions, molecules, and more reside in this ethereal environment known as the Interstellar Medium (ISM). The ISM has fascinated scientists for decades, as at least 200 unique molecules form in its cold, low-pressure environment. It’s a subject that ties together the fields of chemistry, physics, and astronomy, as scientists from each field work to determine what types of chemical reactions happen there.
Now, in the recently published cover article of the Journal of Physical Chemistry A, JILA Fellow and University of Colorado Boulder Physics Professor Heather Lewandowski and former JILA graduate student Olivia Krohn highlight their work to mimic ISM conditions by using Coulomb crystals, a cold pseudo-crystalline structure, to watch ions and neutral molecules interact with each other.
From their experiments, the researchers resolved chemical dynamics in ion-neutral reactions by using precise laser cooling and mass spectrometry to control quantum states, thereby allowing them to emulate ISM chemical reactions successfully. Their work brings scientists closer to answering some of the most profound questions about the chemical development of the cosmos.
Filtering Via Energy
“The field has long been thinking about which chemical reactions are going to be the most important to tell us about the makeup of the interstellar medium,” explains Krohn, the paper’s first author. “A really important group of those is the ion-neutral molecule reactions. That's exactly what this experimental apparatus in the Lewandowski group is suited for, to study not just ion-neutral chemical reactions but also at relatively cold temperatures.”
To begin the experiment, Krohn and other members of the Lewandowski group loaded an ion trap in an ultra-high vacuum chamber with various ions. Neutral molecules were introduced separately. While they knew the reactants going into the ISM-type chemical experiment, the researchers weren’t always certain what products would be created. Depending on their test, the researchers used different types of ions and neutral molecules similar to those in the ISM. This included CCl+ ions fragmented from tetrachloroethylene.
“CCl+ has been predicted to be in different regions of space. But nobody's been able to effectively test its reactivity with experiments on Earth because it's so difficult to make,” Krohn adds. “You have to break it down from tetrachloroethylene using UV lasers. This creates all kinds of ion fragments, not just CCl+, which can complicate things.”
Whether using calcium or CCl+ ions, the experimental setup allowed the researchers to filter out unwanted ions using resonant excitation, leaving the desired chemical reactants behind.
“You can shake the trap at a frequency resonant with a particular ion’s mass-to-charge ratio, and this ejects them from the trap,” says Krohn.
Cooling via Laser to Create Coulomb Crystals
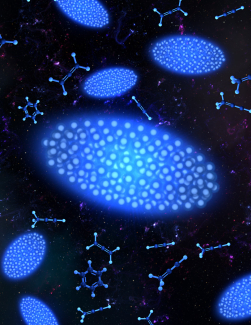
After filtering, the researchers cooled their ions using a process known as Doppler cooling. This technique uses laser light to reduce the motion of atoms or ions, effectively cooling them by exploiting the Doppler effect to preferentially slow particles moving toward the cooling laser. As the Doppler cooling lowered the particles’ temperatures to millikelvin levels, the ions arranged themselves into a pseudo-crystalline structure, the Coulomb crystal, held in place by the electric fields within the vacuum chamber. The resulting Coulomb crystal was an ellipsoid shape with heavier molecules sitting in a shell outside the calcium ions, pushed out of the trap's center by the lighter particles due to the differences in their mass-to-charge ratios.
Thanks to the deep trap that contains the ions, the Coulomb crystals can remain trapped for hours, and Krohn and the team can image them in this trap. In analyzing the images, the researchers could identify and monitor the reaction in real time, seeing the ions organize themselves based on mass-to-charge ratios.
The team also determined the quantum-state dependence of the reaction of calcium ions with nitric oxide by fine-tuning the cooling lasers, which helped produce certain relative populations of quantum states of the trapped calcium ions.
“What's fun about that is it leverages one of these more specific atomic physics techniques to look at quantum resolved reactions, which is a little bit more, I think, of the physics essence of the three fields, chemistry, astronomy, and physics, even though all three are still involved,” adds Krohn.
Timing is Everything
Besides trap filtration and Doppler cooling, the researchers' third experimental technique helped them emulate the ISM reactions: their time-of-flight mass spectrometry (TOF-MS) setup. In this part of the experiment, a high-voltage pulse accelerated the ions down a flight tube, where they collided with a microchannel plate detector. The researchers could determine which particles were present in the trap based on the time it took for the ions to hit the plate and their imaging techniques.
“Because of this, we've been able to do a couple of different studies where we can resolve neighboring masses of our reactant and product ions,” adds Krohn.
This third arm of the ISM-chemistry experimental apparatus improved the resolution even further as the researchers now had multiple ways to determine which products were created in the ISM-type reactions and their respective masses.
Calculating the mass of the potential products was especially important as the team could then switch out their initial reactants with isotopologues with different masses and see what happened.
As Krohn elaborates, “That allows us to play cool tricks like substituting hydrogens with deuterium atoms or substituting different atoms with heavier isotopes. When we do that, we can see from the time-of-flight mass spectrometry how our products have changed, which gives us more confidence in our knowledge of how to assign what those products are.”
As astrochemists have observed more deuterium-containing molecules in the ISM than is expected from the observed atomic deuterium-to-hydrogen ratio, swapping isotopes in experiments like this allows researchers to get one step closer to determining why this may be.
“I think, in this case, it allows us to have good detection of what we're seeing,” Krohn says. “And that opens more doors.”
This work was supported by the National Science Foundation and the Air Force Office of Scientific Research.
Written by Kenna Hughes-Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.