Physics has always been a science of rules. In many situations, these rules lead to clear and simple theoretical predictions which, nevertheless, are hard to observe in actual experimental settings where other confounding effects may obscure the desired phenomena. For JILA and NIST Fellows Ana Maria Rey and Jun Ye, one type of phenomena they are especially interested in observing are the interactions between light and atoms, especially those at the heart of the decay of an atom prepared in the excited state. “If you have an atom in the excited state, the atom will eventually decay to the ground state while emitting a photon,” explained Rey. “This process is called spontaneous emission.” The spontaneous emission rate can be manipulated by scientists, making it longer or shorter, depending on the experimental conditions. Many years ago it was predicted that one way to suppress or slow down spontaneous emission was by applying a special type of statistics known as Fermi statistics which prevents two identical fermions from being in the same quantum state, known as the Pauli Exclusion Principle
This principle is similar to a game of Duck, Duck, Goose, where two individuals fight over an open spot in a circle in order to avoid being “it.” Like children in this game, the atoms must find an empty quantum state to decay into. If they cannot find an empty state, interesting things begin to happen. “If an excited atom wants to decay, but the ground state is already filled, then the decay is “Pauli blocked” and the atom will stay in the excited state longer, or even forever,” Rey said. Nevertheless, the experimental observation of this effect happened to be challenging. It was not until last year that the Ye group observed Pauli blocking of radiation for the first time indirectly by measuring the light scattered by the atoms—but a direct observation of Pauli blocking by measuring the lifetime of atoms in the steady state was lacking. More recently, Ye’s and Rey’s groups collaborated in a joint study, and were able to find an appropriate experimental setting where they were able to observe Pauli blocking of spontaneous emission by direct measurements of the excited state population. The results have been published in the journal Physical Review Letters.
Atomic Decay and Overcoming the Problems with Pauli Blocking
The process of spontaneous emission depends on a variety of things. “In generic terms, the spontaneous emission rate fundamentally is determined by the density of the final accessible states of the atom-light system. This means that the rate can be engineered!” Rey said. “For example, we know that in cavity systems, by putting the atom between two highly reflecting mirrors, we can alter the lifetime of the atoms. In this case, we can make them decay really fast, a phenomenon called superradiance.”
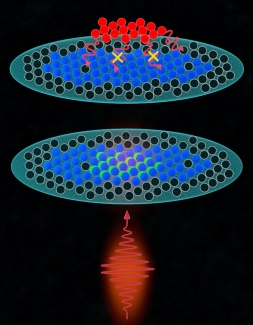
Similarly, there are many ways to make an atom decay at a slower rate. One of these ways is by applying the aforementioned Pauli Exclusion Principle. While Pauli blocking could work in theory, in reality, it is much more difficult to see in a laboratory. This is due to complicating effects and additional interactions the atoms are experiencing that can compete with Pauli blocking. One of these is atomic recoil, essentially a “kick-back” an atom experiences when emitting a photon. This atomic recoil shifts the atom's momentum, allowing it to decay as it now has a (vastly) different momentum with respect to the other present atoms, negating any Pauli blocking fo the atom. The other competing effects are from dipole-dipole interactions, which are forces between pairs of atoms due to exchange of photons. “These interactions are going to modify the decay properties of the atoms and compete with Pauli blocking,” Rey said.
In 2021, Ye performed an experiment to try to observe Pauli blocking prior to the present study. For that experiment, the group used an ultra-cold cloud of fermionic atoms and illuminated it with off-resonant light to excite a few of the atoms in the cloud. According to research associate Thomas Bilitewski: “What they did observe was that in certain directions less light was being observed which they could trace directly to Pauli blocking.” Ye's experiment was able to observe Pauli blocking indirectly for the first time, by focusing on the emitted light instead of looking directly at the excited atoms, which is much harder.
Directly seeing excited atoms live longer hadn’t been demonstrated and that was the goal of the current project. In the former (2021) experiment it wouldn’t have been possible to directly observe the atomic decay easily since the atomic transition they chose was too fast. “If the atom decays too fast, it's going to be very hard to track them,” Rey stated. To accomplish this goal the Ye's group used a transition with a decay time of 22 microseconds. This timescale is perfect for directly observing the Pauli blocking enhanced lifetime, as it is slow enough to make the observation possible. However, even if this atomic transition makes a direct observation of Pauli blocking possible in principle, one still has to understand the role of the competing effects and interactions to make a clear case for the observation of Pauli blocking.
Atomic Kick-Backs
Bilitewski was particularly interested in the Ye group's experiments. Many of the initial results came from a 3D gas cloud, but after seeing little evidence of Pauli blocking, the Ye group switched to a 2D compressed gas cloud. The switch from 3D to 2D inspired Bilitewski to work on a theoretical model for Pauli blocking in the 2D plane. “In a 2D system, we can avoid the initial momentum kicks [atomic recoil] from the laser exciting the atoms,” explained Bilitewski. “We can prevent that initial kick [and change in momentum of the atom] by tight confinement, so you get a stronger Pauli blocking.”
This process of Pauli Blocking can be leveraged to the researcher's advantage. Rey, Bilitewski, and their team also used existing capabilities within the Ye group's experiment to fine-tune how the atoms were excited. “We considered a multi-level system where effectively we have two ground state levels and one excited level,” Bilitewski said. “Importantly, you can choose to only excite one of the two ground states by choosing the proper laser polarization. This, together with the capability to control the initial population of the two ground states, allows you to independently tune how many blocking atoms are in the system and how many atoms you excite. So, you can tune the strength of Pauli blocking and dipolar interactions independently” This setting allowed the researchers to have better control over their experimental system, and led to a clear signature of Pauli blocking.
To describe the multi-level system in the 2D geometry, the researchers developed a theoretical model that would account for all the effects happening during the dynamics that might affect Pauli blocking. “It's a bit complicated to account for all these effects at the same time,” Bilitewski added. “And as is often the case, you have to make some approximations to make the problem tractable. In the end, it's about finding the sweet spot where you are still describing what happens in the experiment, but can actually do the calculation, and it turned out to work beautifully.” The team is hoping that future experiments will test their theoretical model beyond the regime where it was shown to describe the current model and to demonstrate even stronger Pauli blocking in the laboratory under new conditions, including colder temperatures. “It's an open question whether Pauli blocking will persist in a more strongly correlated setting,” Rey said. “This is a very exciting direction to research in the future.”
Written by Kenna Castleberry, JILA Science Communicator.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.