When illuminated by X-ray and infrared light beams in tandem, electrons can tap dance off a platinum surface because they've actually grabbed a photon from both beams simultaneously. As you might have guessed, there is more going on here than the ordinary photoelectric effect, which Albert Einstein explained more than a century ago. In the photoelectric effect, electrons escape from a solid after absorbing a single photon or bundle of light energy. What happens when two laser beams simultaneously hit a surface is called the "laser-assisted photoelectric effect."
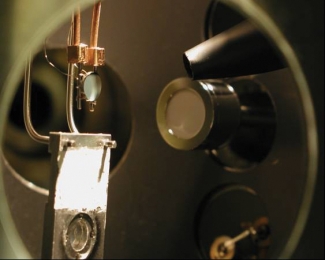
The laser-assisted photoelectric effect was recently observed for the first time in a solid by research associate Guido Saathoff, graduate student Luis Miaja-Avila, Fellows Margaret Murnane and Henry Kapteyn, former post-doc Chi-Fong Lei, and former Visiting Fellows Martin Aeschlimann (Technische Universität Kaiserlautern) and John Gland (University of Michigan). Their apparatus is shown at right. A platinum sample (the disk at the left of center) is simultaneously illuminated by X-ray and infrared (IR) laser beams. In addition to absorbing the X-ray photon energy necessary for escaping from the surface, the electrons escaping from the surface can simultaneously either grab, or hurl away, an infrared photon. This complex interaction changes the characteristic energies of the newly freed electrons.
The laser-assisted photoelectric effect works the same way on a surface as it does in a cloud of atoms: energetic X-rays eject electrons from a surface (or a cloud of atoms). The liberated electrons would normally have a fixed energy, given by the absorbed photon energy minus the energy needed to escape the surface barrier. This is simply Einstein's photoelectric effect. However, when an intense IR pulse hits the surface at the same time as an X-ray pulse, the liberated electrons are either sped up or slowed down as they escape the surface barrier. Which way it goes depends on the exact moment in the IR light wave when it encounters a particular free electron.
What's interesting is that the Murnane-Kapteyn group didn't set out to discover the laser-assisted photoelectric effect on a surface. In fact, until the group discovered it, physicists did not realize that it could be observed in solids. Rather, the researchers had intended to probe molecular vibrations on a surface that had well-defined energy states. However, when they simultaneously illuminated their surface with IR and X-ray beams, the well-defined photoemission energy structure, which is usually observed from a platinum surface, completely disappeared. In looking for an explanation for this unexpected result, they deduced that the laser-assisted photoelectric effect was the only process that could explain their observations. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.