The Ye group just solved a major problem for using molecular fingerprinting techniques to identify large, complex molecules: The researchers used an infrared (IR) frequency comb laser to identify four different large or complicated molecules. The IR laser-light absorption technique worked well for the first time with these larger molecules because the group combined it with buffer gas cooling, which precooled their samples to just a few degrees above absolute zero. This innovative combination is expected to provide insights into the physics of molecular structure and behavior. It will also improve trace gas detection techniques used in breath analysis, hazardous gas identification, atmospheric chemical analysis, and climate science studies.
The reason even medium-sized molecules previously posed a problem in molecular fingerprinting is that all the atoms in molecules rotate and vibrate in complex patterns. And the more atoms there are moving wildly and communicating among themselves, the more spectral lines crowd into one spectrum. The spectrum becomes so congested it cannot be analyzed. This congestion occurs because even medium-sized organic molecules (containing carbon, hydrogen, and oxygen) typically have millions of rotational-vibrational states at room temperature. These states all show up in a single IR spectrum. And, the problem with congested spectra gets even worse as molecules get bigger and bigger.
The researchers who solved the problem of analyzing large, complex wild molecules include research associates Ben Spaun and Oliver Heckl, graduate students Bryan Changala and Bryce Bjork, Fellow Jun Ye and their colleagues Dave Patterson and John Doyle from Harvard University.
Spaun and his team were well positioned to make this breakthrough. The Ye group figured prominently in the development of the original laser frequency comb, or ruler of light; the group also developed the first mid-IR laser frequency comb. The mid-IR comb consists of thousands of evenly spaced spectral lines that can be used to detect, measure, and identify unknown substances with exquisite accuracy––as long as the spectrum isn’t too congested.
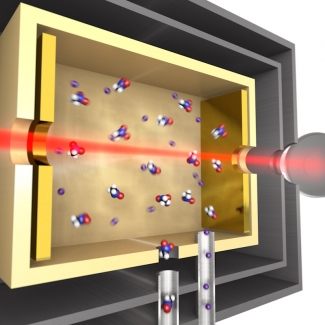
In this breakthrough experiment, researchers first cooled molecules of nitromethane (7 atoms), naphthalene (18 atoms), adamantine (26 atoms), or hexamethylenetetramine (22 atoms). Then the researchers placed the molecules in a hollow chamber with comb light bouncing back and forth through the molecules. The molecules absorbed the comb light at the specific frequencies at which the molecules rotate and vibrate. These absorption patterns, which are unique for each different molecule, are the molecular fingerprints that made their identification possible.
Identifying these large, complex molecules also required the use of an innovative cooling technique known as buffer-gas cooling, which was developed by Patterson and Doyle. This technique used a small commercial liquid helium refrigerator cooled to 4 K (just 4 degrees above absolute zero). First, the researchers flowed helium atoms into the refrigerator. These atoms collided with the walls of the refrigerator, which cooled them to 4 K.
Second, the researchers introduced some their “hot” test molecules to the refrigerator. The test molecules collided with the helium atoms, which transferred heat from the test molecules into the walls of the refrigerator. Once the test molecules got very cold, their vibrations and rotations calmed way down. The molecules now moved very slowly through the hollow chamber where the comb light was bouncing back and forth between two mirrors.
The wild molecules were tamed, and the molecular fingerprinting process worked like a charm!


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.