Falling on ice is never fun. A cold and slippery rink is as unforgiving as concrete. Falling into snow, however, is like falling into a pile of leaves. Fluffy, sometimes sticky, and soft enough to be the only substance societally acceptable to throw at another person’s face, snow couldn’t be more different than ice. But they’re both solid H2O, they’re just in different states.
JILA researchers in the Kapteyn-Murnane group recently discovered the insulator version of snow. Using a new ultrafast electron calorimetry technique, the team discovered a never-before-seen state in an otherwise standard semiconductor. This state is insulating, yet its properties differ from the material’s standard insulating state. This research, published on March 1st in Science Advances, is the group’s most recent demonstration of using ultrafast lasers to uncover new understandings—and manipulate properties—of well-known materials.
Hot in a Femtosecond
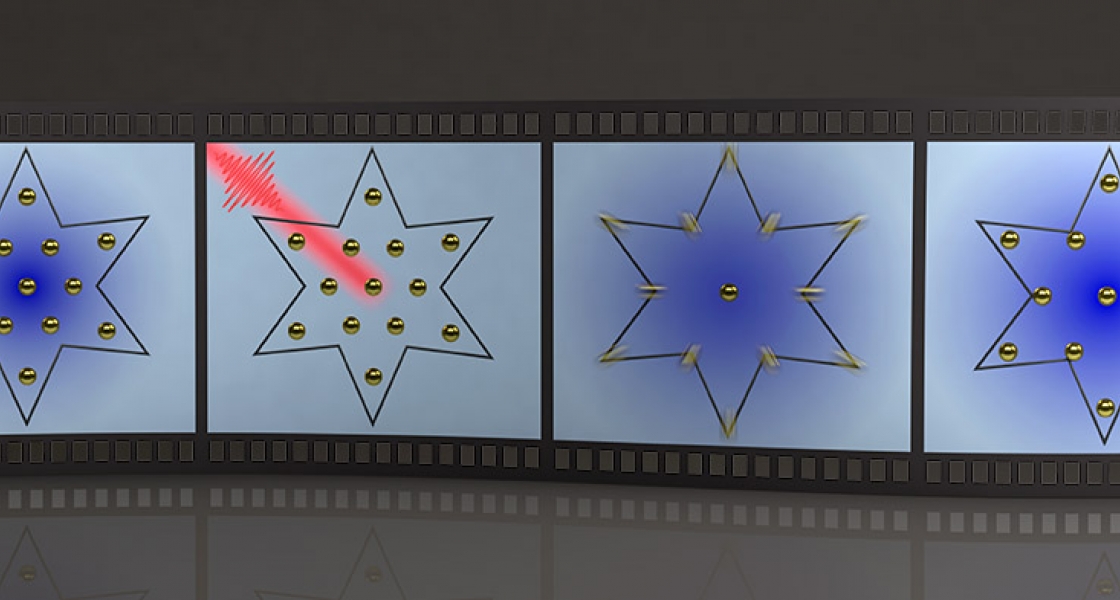
Tantalum diselenide (TaSe2) is a semiconductor built from one layer of atomically thin tantalum sandwiched between two layers of selenide. When cold, the atoms in this material bunch together into a star shape. This shape shackles the electrons to the atoms and impedes the flow of electricity, therefore rendering the material as an insulator. But warm this material and insulator melts into a conductor like ice melting to water.
Warm it quickly, however, and the insulator jumps into a previously-unknown insulating state. And by quick, they mean quick—the Kapteyn-Murnane team boosted the temperature tantalum diselenide’s electrons more than 3000 Kelvin in just a few femtoseconds, or just a few millionths of a billionth of a second.
To jump the temperature this quickly the team used an ultrafast laser. According to JILA Fellow Dr. Margaret Murnane, a principal investigator of this experiment, such a fast speed ensures that the ultrafast laser heats only the electrons and not the atoms.
“The laser excites the electrons and smears them spatially. And now the insulator is destabilized, so the atoms, instead of being bunched up in a star, move away from each other to try to be equally spaced. But as the atoms rearrange, the material suddenly enters a new state that was previously hidden,” said Murnane.
While this new state is still insulating, it has a much lower heat capacity than the usual insulating state. This means that the material requires much less energy to switch between states.
“A phase where the heat capacity drops to a third of its normal value—that’s very surprising,” said Murnane
Gone in a Nanosecond
Dr. Xun Shi, the corresponding author of this research, mentioned another surprising find. “One reason that no one found this new phase is that it is not stable under normal equilibrium conditions… but it is metastable.”
A metastable state is one with a lifetime, like snow on a warm sidewalk. This particular state lasts about 1 nanosecond, or a billionth of a second, before it drops back into the usual insulating state, said Shi. But despite how short this sounds, Shi assured that this is quite a long time in material science.
Long enough, in fact, for this state to have applications in computing and electronics. Ultrafast switches, for example, could be flipped within femtoseconds by using laser-induced state changes like the one found here.
The discovery of this state would not have been possible without the KM group’s ultrafast electron calorimetry technique. This technique, in which extreme ultraviolet (EUV) light probes the temperature of electrons, previously enabled JILA researchers to study electron dynamics in magnetic materials and discover that electrons react ten times faster than previously thought.
“The [ultrafast electron calorimetry] technique is a way to systematically map when there is a change in phase and microscopic interactions in the material,” said Murnane. “Not all kinds of interactions, but important ones for quantum materials that will be the basis of next-generation nanotechnologies.”
Just as snow is different from ice, the team expects to find surprising differences between this new insulating state and the standard one. Thus far, the team has discovered that by tuning the laser power they can affect the lattice distortion, thus enabling them to study the properties of this state. In the future, the group plans to further study these interactions, specifically aiming to understand why the electrons preferentially couple to some lattice vibrations and not others. And looking beyond tantalum diselenide, they plan to use this same technique to explore and control new states in more materials.
Minding all their lofty goals, Murnane summarizes it well. “Learning how to manipulate a material with light, that’s what we’re trying to do.”
In addition to JILA postdoc Xun Shi, this research was conducted by graduate students Wenjing You, Yingchao Zhang, JILA postdoc Dr. Zhensheng Tao, as well as Dr. Peter M. Oppeneer of Uppsala University, Dr. Xianxin Wu and Dr. Ronny Thomale of Julius-Maximilians-Universität Würzburg, and Dr. Kai Rossnagel and Dr. Michael Bauer of Kiel University. The Principal Investigators were JILA Fellows Dr. Margaret Murnane and Dr. Henry Kapteyn. This research was published in Science Advances on 1 March 2019.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.