Understanding the chemical and physical properties of surfaces at the molecular level has become increasingly relevant in the fields of medicine, semiconductors, rechargeable batteries, etc. For example, when developing new medications, determining the chemical properties of a pill's coating can help to better control how the pill is digested or dissolved. In semiconductors, precise atomic level control of interfaces determines performance of computer chips. And in batteries, capacity and lifetime is often limited by electrode surface degradation. These are just three examples of the many applications in which the understanding of surface coatings and molecular interactions are important.
The imaging of molecular surfaces has long been a complicated process within the field of physics. The images are often fuzzy, with limited spatial resolution, and researchers may not be able to distinguish different types of molecules, let alone how the molecules interact with each other. But it is precisely this–molecular interactions–which control the function and performance of molecular materials and surfaces.
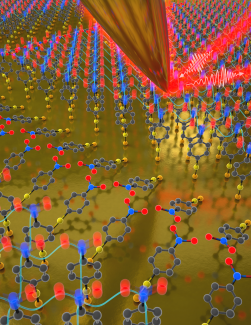
In a new paper published in Nano Letters, JILA Fellow Markus Raschke and graduate student Thomas Gray describe how they developed a way to image and visualize how surface molecules couple and interact with quantum precision. The team believes that their nanospectroscopy method could be used for molecular engineering to develop better molecular surfaces, with controlled properties for molecular electronic, photonic, or biomedical applications.
Imaging Monolayers with an Infrared Nano-eye
In order to test their new nanospectroscopic imaging method, the researchers used a so called self-assembled monolayer of small organic molecules of 4-nitrothiophenol. The monolayer was then placed under the tip of an atomic force microscope (AFM). Gray explained the process-: "We used infrared light, which has a very long wavelength, limiting spatial resolution to the order of microns, or thousands of molecules across. The way we get the nanometer spatial resolution is using the extremely sharp tip of an atomic force microscope, which is only tens of molecules across. It acts as a lightning nod, just for light, and can focus it to the nanoscale. This allows us to image and perform spectroscopy on the nanoscale with sensitivity as high as just a few molecules." Gray emphasized that because of its low energy, the infrared light directly probes molecular structure, as it could indicate if the 4-nitrothiophenol molecules interacted or coupled with each other.
Molecular Interactions and Quantum Sensors
Principal investigator Raschke was excited about seeing these molecular interactions, and postulated that these interactions could be used for quantum sensing. In order to test their hypothesis of successful quantum sensing, the team looked at the coupling to determine the size of the surface domains. Gray categorized the type of coupling as "a vibrational exciton delocalized across many molecules.” He added that: “When people hear the term exciton they think of electronic excitations. Our vibrational exciton is the conceptual analogue just for molecular vibrations." Using their new imaging systems, the team could see these vibrational excitons on their natural length scales extending across just a handful of molecules. Raschke explained that their nanolocalized infrared light of their imaging system improved their view of these excitons because "the tip itself already provides localization and spatial resolution down to a few tens of nanometers–that is already one ten-thousandth [the width of]of a human hair. The vibrational exciton quantum sensor provides another improvement of spatial resolution by a factor of ten into the true molecular scale. And with a sensitivity where we can distinguish if two, three, or four molecules would be interacting, meaning sharing their wavefunction, or colloquially, speaking to each other in a quantum sense." This new nanospectroscopic approach of vibrational exciton nanoscopy could be used to improve and engineer molecular materials from the from the start and better predict their properties.
Should the vibrational excitons become a successful quantum sensor, this might have implications for quantum technology more generally. "Some people have theorized quantum state transfer for quantum information applications based on these vibrational excitons," Gray stated. "And the reason it would be nice is because a vibrational exciton is potentially stable at room temperature and you wouldn't have to go down to these low temperatures typically required for quantum sensing or computing." Taking quantum technology out of the cold temperatures usually required for most quantum devices would make them more affordable and more widely accessible.
Having found success with their new imaging system, by taking advantage of vibrational excitons as a super-resolution imaging quantum sensor, Gray and Raschke are expanding their research focus. "We are now extending this work from molecular monolayers to molecular crystals used, for example, in molecular electronics or in light-emitting diodes," said Gray. While this new imaging system is helping to improve quantum technology it is also expanding knowledge about the interactions of molecules at the quantum level thatwill help in designing, improving, and controlling molecular materials in general.
Written by Kenna Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.