Far-red fluorescent light emitted from proteins could one day illuminate the inner workings of life. But before that happens, scientists like Fellow Ralph Jimenez must figure out how fluorescent proteins’ light-emitting structures work. As part of this effort, Jimenez wants to answer a simple question: How do we design red fluorescent proteins to emit longer-wavelength, or redder, light?
The reason for working on this problem is that far-red light emitted by fluorescent proteins would be more useful for “seeing” into the intact organs of live animals such as mice. Far-red light more readily passes through living tissue than do green or blue wavelengths, as those people who have covered a flashlight with their hands can attest.
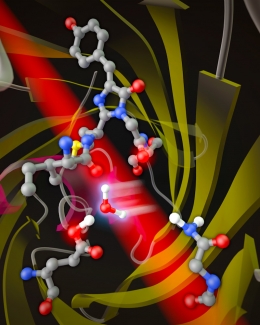
There are two basic approaches to making red fluorescent proteins redder, according to Jimenez. The first one is to change the structure of the small group of atoms called the chromophore that absorb and emit light. In the past, researchers thought that that the longer-wavelength emission from red fluorescent proteins was due to a particularly strong interaction between specific atoms in the chromophore (known as acylimine) and atoms in the barrel-shaped protein surrounding the chromophore. This interaction supposedly gave the electrons in the chromophore more room to move around, which lowered the energy of the photons absorbed and emitted by the chromophore.
The second approach is to fine-tune the motions of the barrel around the chromophore. This approach is favored by the Jimenez group, which studied a protein called mPlum that emits the longest-wavelength red light of any of “mFruit” family of fluorescent proteins. The Jimenez group’s experiments show that mPlum’s redder emission is due to the flexibility of interactions between the barrel and chromophore’s acylimine atoms.
“Water is available in and around the barrel, and water can pop in and pop out as the (floppy) side chain rotates,” Jimenez explained. “This rotation is correlated with the red shift.” In other words, after mPlum absorbs a photon, a water molecule gets in between the chromophore and a sidechain of the protein, causing the chromophore to fluoresce red rather than orange light.
The Jimenez group recently measured this process in detail and determined that after the mPlum’s chromophore is excited with a short pulse of laser light, it takes precisely 37 picoseconds (10-12 s) to convert from a structure without water to a lower-energy structure containing a water molecule.
Following this enlightening experiment, the group collaborated on an analysis of a fluorescent protein known as TagRFP675, which emits even redder light than mPlum. TagRFP675 has two different interactions between the acylimine group of its chromophore and the protein barrel, both of which can interact with water and with other protein structures, with everything in constant motion. The question was whether the two interactions in TagRFP675 responsible for the redder emission occurred via the same mechanism identified for mPlum.
“Two interactions turned out to be too much of a good thing,” Jimenez said, adding that the system is so complex that it emits light from multiple structures simultaneously, and it’s difficult to nail down which ones are responsible for the reddest emission.
“We discovered that there may be an avenue to making a more red-shifted fluorescent protein by learning how to lock down, or immobilize, one of the structures in TagRFP675,” he said.
Jimenez worked on the mPlum and TagRFP675 projects with recently minted JILA Ph.D. Patrick Konold, graduate student Samantha Allen, and colleagues from Pohang University of Science and Technology (South Korea), Florida International University, Virginia Tech, the Massachusetts Institute of Technology, and Albert Einstein College of Medicine.––Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.