Life requires energy, and nature has perfected systems for creating and transporting energy inside a living thing. These incredible feats are possible because of interactions between molecules—particularly interactions between types of molecules called porphyrins.
“Porphyrins are important for energy conversion and transport,” explained Thomas Gray, a graduate student in the Raschke Group at JILA. “Your body uses it to transport energy and oxygen, and plants use it in photosynthesis.”
Molecular interactions—including coupling—are like nature’s secret language, said JILA Fellow Markus Raschke—and it’s a language that scientists are continually working to decipher.
“We are made out of molecules, so the way we function is this coupling, the interaction between molecules. There is beauty in these molecular interactions that define life,” Raschke said. Molecular coupling is also the basis for a lot of molecular electronics, and technologies that replicates living systems, such as photovoltaic solar panels, he added.
The most common tools to study molecular coupling don’t get a high enough resolution to study this phenomenon, and “you really have to look down into the molecular scale to see it”, Raschke said.
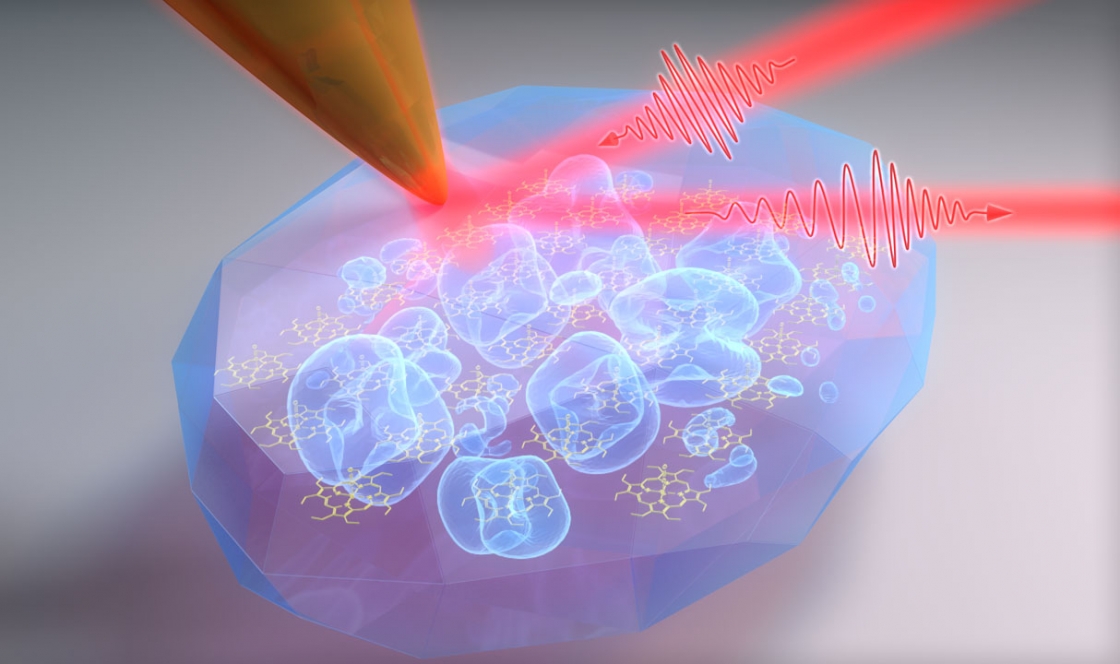
Now the Raschke Group has developed the tools to see this coupling with a high spectral and spatial resolution. In a recently published study in the Proceedings of the National Academies of Sciences, the Raschke Group at JILA focused infrared lasers to an incredibly small spot using a scanning probe microscope to study vibrational excitons, and watch how porphyrin molecules form functional, well-ordered crystals with unprecedented high resolution.
“The power of this is to see really small things,” Gray said. “We have high spectral and spatial resolution. This is some of the highest resolution we have ever gotten.”
Reading nature’s secrets
Studying molecular interactions has been really tricky, Raschke said. Porphyrin molecules are tiny—only a few billionths of a meter long, thousands of times smaller than a human red blood cell. Using X-rays, electron microscopes and high-powered lasers, scientists have been able to see and study the smallest building blocks of the universe, such as atoms.
But those tools weren’t sufficient for this task. Molecular structures are delicate, and high-energy X-rays or electron microscopes can warp and distort the molecular interactions. Infrared light, however, is much gentler, Raschke explained; after all, we interact with infrared radiation all the time. We feel its warmth, but we don’t get sunburn.
“The feature of the infrared light is that it is very minimally perturbing,” he said. It interacts with delicate molecular structures without damaging them.
When exciting the molecule, the infrared light can specifically sense the intermolecular interaction. But the wavelength of infrared light is very long—10,000 times larger than molecule dimensions.
To overcome this problem, the Raschke group used a trick to focus to the light to the right size. They use ultra-sharp tips made out of gold, with a tiny apex only a few nanometers in size. These tips act just like an antenna for infrared light and can focus it down to 1/1000th of its wavelength.
“This is similar to a lightning rod, just for infrared light,” Gray explained.
Then the scanning probe microscope acts like just like the needle in an old-school record player, Raschke said. Moving the tip across the sample with the porphyrin nano-crystals, the tip probes the molecules, and sends data and images back to the physicists.
“You are reading the secrets of nature,” he said. “You can read what you cannot access with the unaided eye.”


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.