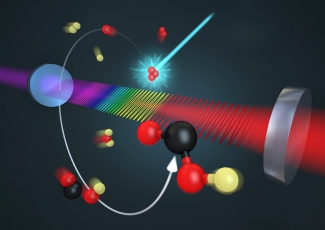
Using frequency comb spectroscopy, the Ye group has directly observed transient intermediate steps in a chemical reaction that plays a key role in combustion, atmospheric chemistry, and chemistry in the interstellar medium. The group was able to make this first-ever measurement because frequency combs generate a wide range of laser wavelengths in ultrafast pulses. These pulses made it possible for the researchers to “see” every step in the chemical reaction of OH + CO → HOCO → CO2 + H.
This reaction is an example of the importance of free radicals such as the hydroxyl radical (OH), which has an unpaired electron that makes it highly reactive. Understanding (and one day controlling) the reaction OH + CO → HOCO → CO2 + H will lead to a better understanding of combustion processes as well as atmospheric chemistry and greenhouse gases. In the atmosphere, for example, the reaction of OH with CO adds carbon dioxide (CO2) to the atmosphere when fossil fuels are burned.
Here on Earth, Bryce Bjork, Thinh Bui, and their colleagues in the Ye group used frequency comb spectroscopy to observe the detailed intermediate steps of the full reaction of OH with CO for the first time. The researchers used one trick to make their job easier. They substituted deuterium (D), or heavy hydrogen, for the H in the OH.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.