Benzene has a special ring structure that allows some of its electrons to be shared among all six carbon atoms in the ring. It turns out that chemists like Fellow J. Mathias Weber can adjust the charge density in the ring by exchanging hydrogen (H) atoms in the ring with other atoms or groups of atoms. Such exchanges can change the charge pattern in the ring "seen" by neighboring molecules.
The interaction based on the charge distribution isn’t the whole story, however. Often, negatively charged atoms or molecules (generally called anions) can link to a carbon-hydrogen (CH) group in a benzene molecule by hydrogen bonding. Hydrogen bonding is an attractive interaction between an H atom in one molecule and a negatively charged atom, such as oxygen or fluorine, in another molecule. It is based not only on the attraction between opposite charges, but also — like many chemical-bonding phenomena — on the partial transfer of electrons from the negatively charged bonding partner to the molecule that contains the H atom.
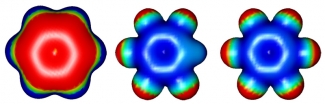
Graduate students Holger Schneider and Kristen Vogelhuber recently investigated how anions bind to benzene molecules as they successively exchange H atoms in the ring for fluorine (F) atoms. "Normal" benzene (C6H6) is negatively charged inside the ring, and positively charged on the outside. The F atoms "pull" negative charge out of the ring. As more F atoms are added, the polarity of the ring changes to positively charged on the inside and negatively charged on the outside for hexafluorobenzene (C6F6), as shown in the figure.
While the polarity change starts after the addition of only a few F atoms, the JILA researchers found that as long as there remains even just one H atom in the ring, H bonding will be the preferred mode of interaction between an anion and the fluorinated benzene; all six H atoms have to be exchanged for F atoms for the anion to bind to the ring itself.
This behavior intrigues chemists who want to use benzenelike parts of one molecule as binding sites for negatively charged groups on another molecule to make "supramolecular" structures. Such structures can assemble themselves into well-ordered thin films or nanomaterials with entirely new chemical properties. - J. Mathias Weber


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.