Science sleuths have a new and powerful method for identifying (and investigating) atoms and molecules, thanks to Graduate Student Mike Thorpe, Research Associate Kevin Moll, Senior Research Associate Jason Jones, Undergraduate Student Assistant Ben Safdi, and Fellow Jun Ye. The new method allows them to study molecular vibrations, rotations, and collisions as well as temperature changes and chemical reactions. The technique can also detect trace amounts of chemicals with exquisite sensitivity because it can precisely identify their characteristic patterns of laser light absorption, or molecular fingerprints. What's so special about this method is that its broad spectral bandwidth makes it possible to detect millions of parallel light channels simultaneously in real time.
In addition to being a powerful research tool, the new technique has some promising applications. For example, medical doctors will be able to use it to diagnose illnesses by detecting telltale chemicals in a simple breath analysis. Security screeners will be able to use it to identify potential hazards such as explosives, toxic gases, or dangerous pathogens. Mike Thorpe is already working on developing a practical device that will be smaller, less expensive, and easier to operate than the research prototype. Naturally, Ye's group has applied for patents on their new system.
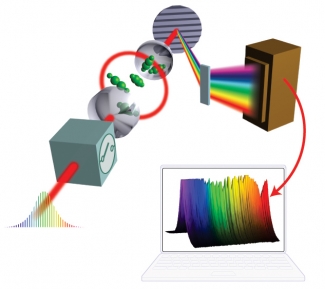
The diagram on the right outlines how the method works: First, an ultrafast laser creates a wide bandwidth optical frequency comb. The comb includes a multitude of precisely known frequencies in the visible and near infrared region of the electromagnetic spectrum.
Second, the comb is coupled to an optical cavity containing one or more molecules. Coupling ensures that the light in the cavity has the same precise frequencies as the comb.
Third, chemicals inside the cavity absorb photons of particular frequencies (which are determined by each chemical's electronic, vibrational, and rotational structure). The pattern of photon absorption is unique for every different kind of atom or molecule.
Finally, light exiting the cavity is analyzed to see not only what frequencies of light are missing photons, but also how many photons of a particular frequency have disappeared. Since the pattern of missing photons is unique for each chemical, the researchers can identify the components of a mixture by comparing the mixture's photon absorption pattern with known molecular fingerprints of possible individual constituents.
The work reported here appeared in the March 17, 2006, issue of Science. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.