Although one might think it would be simple, the genetics of bacteria can be rather complicated. A bacterium’s genes use a set of regulatory proteins and other molecules to monitor and change genetic expressions within the organism. One such mechanism is the riboswitch, a small piece of RNA that can turn a gene “on” or “off.” In order to “flip” this genetic switch, a riboswitch must bind to a specific ion or molecule, called a ligand, at a special riboswitch site called the aptamer. The ligand either activates the riboswitch (allowing it to regulate gene expression) or inactivates it until the ligand unbinds and leaves the aptamer. Understanding the relationship between ligands and aptamers can have big implications for many fields, including healthcare. “Understanding riboswitches and gene expression can help us develop better antimicrobial drugs,” explained JILA graduate student Andrea Marton Menendez. “The more we know about how to attack bacteria, the better, and if we can just target one small interaction that prevents or abets a gene from being translated or transcribed, we may have an easier way to treat bacterial infections.”
To better understand the dynamics of aptamer and ligand binding, Marton Menendez, along with JILA and NIST Fellow David Nesbitt, looked at the lysine (an amino acid) riboswitch in Bacillus subtilis, a common type of bacterium present in environments ranging from cow stomachs to deep sea hydrothermal vents. With this model organism, the researchers studied how different secondary ligands, like, potassium, cesium, and sodium, affect riboswitch activation, or its physical folding. The results have been published in the Journal of Physical Chemistry B.
Pairing Up Molecules
“We know that cells are complicated; living systems are really complicated,” Marton Menendez stated. “There's a lot going on in them. But when we're trying to study complicated processes, such as how exactly does DNA or RNA fold? we tend to simplify a lot. So, we usually end up reducing the system down to the simplest DNA/RNA structure we want to study and a few necessary salts.” With this idea in mind, Marton Menendez and Nesbitt analyzed their bacterial system using single molecule FRET (fluorescence resonance energy transfer) microscopy. This type of microscopy uses pairs of fluorescent dye molecules to tag specific nucleic acid positions, for this study in particular, a larger RNA riboswitch, allowing researchers to study binding, folding, and unfolding in real time
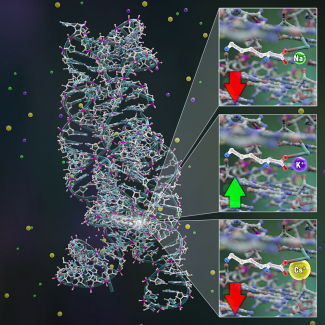
For this particular riboswitch to work, lysine first binds to the aptamer, which causes the aptamer to fold around lysine. However, in the x-ray crystallography images of the riboswitch, a potassium ion was also bound in the aptamer. According to Marton Menendez: “You can take crystal structures of these pieces of RNA and analyze their content. If the something shows up in the crystal structure, like the potassium ion, it is likely to have been very tightly bound in the riboswitch, because it means that it stayed there a long time. This tells us that potassium can play a ligand-like role for our riboswitch.”
Besides studying potassium as a potential ligand, the researchers also found that when potassium was bound to the riboswitch, it changed how the riboswitch interacted with lysine, the primary ligand. “We looked at how the riboswitch functions with respect to lysine and potassium because they affect each other,” Marton Menendez said, “mainly potassium can tweak some of the lysine’s binding abilities. That's interesting because we think of riboswitches as extremely specific and working only with one specific target molecule.” Instead, in the B. subtilis system, this riboswitch interacts with both lysine and potassium, cooperatively, with the presence of one species enhancing the impact of the other.
A Complex Bacterial Evolution
The idea of RNA regulating its own gene expression suggests that the history of bacterial genetic evolution is more complicated than expected. “If you are an early bacterium, how do you regulate your own genes?” Marton Menendez explained. “"There is a hypothesis that the ancient world had only RNA, no proteins or DNA. So RNA alone was responsible for gene storage and regulation. Riboswitches are an example of how RNA can perform these regulatory functions without protein assistance.” As proteins and more complicated organisms emerged, it is easy to expect these genetic systems to evolve to being more complicated, with a larger number of genes and corresponding regulatory proteins. However, results like Marton Menendez’s and Nesbitt’s suggest that there is more in the bacteria’s genes than meets the eye.
With a more complicated relationship between ligands and aptamers, Marton and Nesbitt were interested to see if this relationship could be found in other bacteria, not just B. subtilis. “There's also a version of a lysine riboswitch that exists in bacteria that live in habitats that are at 80 degrees Celsius, near hydrothermal vents on the sea floor,” elaborated Marton Menendez. “We are preparing a paper comparing how regulation by the lysine riboswitches differs between the two bacteria.”
More Complicated and Cooperative Ligand Relationships
Curious about the flexibility in ligand binding to their aptamer, Marton Menendez and Nesbitt decided to see just how versatile the aptamer could be. “We were also interested to see if potassium ion could then be swapped out for something similar,” Marton Menendez added. “The reason the riboswitch goes for lysine might have something to do with the fact that you've got potassium in the system. But, if you have something that's bigger or smaller than potassium, the riboswitch may have higher or lower binding affinity to lysine.” This experiment suggested an additional project looking at how closely connected the potassium and lysine were as ligands, and also to see if the aptamer would bind to other potential ligand-cation combinations of different sizes. Cations are small positive molecules that organic systems use to regulate different molecular processes.
As Marton Menendez said: “We studied the size effects of ions binding to the riboswitch. The riboswitch typically binds lysine with potassium, so we tested cesium and sodium ions [common molecules within the bacterium] instead of potassium. However, it seems that cesium might be too big and sodium too small to allow lysine to bind properly.” Analyzing the data, the researchers found that the aptamer was quite specific with respect to choice of cation preferentially binding to potassium and lysine as the “perfect Goldilocks combination of sizes.” Most importantly, this finding suggests that riboswitch activity can be regulated with vastly more flexibility by responding cooperatively to more than a single ligand species concentration at a time. This cooperativity is a trick that Nature has long exploited for increasing functionality of proteins (e.g., oxygen bonding to hemoglobin in red blood cells), so it would seem an entirely plausible strategy for nucleic acids as well.
Written by Kenna Hughes-Castleberry, JILA Science Communicator


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.