The Weber group wants to understand how the individual building blocks of DNA interact with ultraviolet (UV) light. Such knowledge would be an important step toward gaining a detailed understanding of the molecular processes responsible for the UV-induced DNA damage that results in mutations and can lead to cancer or cell death.
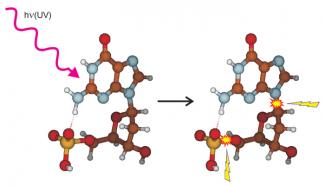
Graduate student Jesse Marcum, student assistant Amit Halevi, and Fellow J. Mathias Weber recently studied the UV photodissociation of DNA subunits, called nucleotides. Nucleotides contain one of the four DNA bases (adenine, cytosine, guanine, or thymine) linked to a sugar molecule, which, in turn, is linked to a phosphate group. In DNA strands, the phosphate and sugar groups of many individual nucleotides are chemically linked together to form the DNA backbone.
The researchers found that the UV light, which was absorbed by the four bases, causes chemical reactions inside the molecule that can break chemical bonds in two places in the nucleotides. First, the break can occur in the link between the phosphate and sugar groups. In an extended DNA chain, this sort of damage would lead to breaking of the DNA strands. Second, the bond can break between the sugar and the base; this breakage can be followed by additional reactions and lead to the loss of genetic information from DNA. Both types of UV damage can occur in living tissue and require enzymes for repair. However, the processes responsible for this damage inside molecules are not completely understood.
Interestingly, the molecules break apart at the same two places following collisions between nucleotides and gas atoms. However, the likelihood of each process occurring was quite different than in UV photodissociation. A comparison of breakage caused by collisions with that caused by UV light suggested that both processes lead to strongly vibrating, i.e., hot, ground-state ions. These ions then fall apart at one of two vulnerable bonds after the vibrational energy gets distributed throughout the molecule.
However, because of the differences in the break-up patterns between collision-induced and photo-induced dissociation, Marcum and his colleagues questioned whether the mechanisms were the same. In collision-induced processes, energy gets deposited in the nucleotide at random positions, while the absorption of UV light by a nucleotide always happens in the base. This means that the precise way in which the molecule is “heated up” is likely to be different after a collisional excitation than after a UV excitation. In other words, the vibrational energy is distributed in a different way through the molecule.
The researchers concluded that although the details of vibrational relaxation are still unclear, the energy cannot be completely randomized in both processes. They plan additional experimental work in the near future aimed at identifying the correct mechanisms. They also want to work with theoreticians to better understand how energy is redistributed in nucleotides when they go from electronically excited states back to their ground states. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.