The Perkins Group has demonstrated a 50-to-100 times improvement in the time resolution for studying the details of protein folding and unfolding on a commercial Atomic Force Microscope (AFM). This enhanced real time probing of protein folding is revealing details in these complex processes never seen before. This substantial enhancement in AFM force spectroscopy may one day have powerful clinical applications, including in the development of drugs to treat disease caused by misfolded proteins. Misfolded proteins are implicated in such fatal maladies as Creutzfeldt–Jakob disease and mad cow disease, both of which are caused by prions.
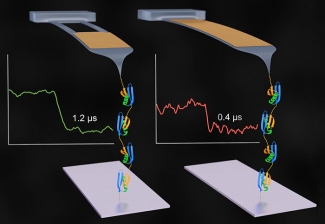
The Perkins Group has been continually improving AFM as a powerful tool to understand the structure and function of proteins through force spectroscopy, measuring the tiny changes in forces as proteins fold and unfold. These folding processes lead to protein function. To get better real-time force spectroscopy, Devin Edwards made a significant breakthrough in optimizing AFM cantilevers for force spectroscopy. The cantilever is the diving-board-like structure that pulls on the proteins.
The first step for Edwards and his colleagues was to start with very small, commercially available cantilevers that would respond quickly to changes in protein structure. The key advancement was to substantially reduce the tendency of these small cantilevers to bounce up and down, or ring, in response to normal random movements of the surrounding liquid molecules (known as Brownian motion). To do so, the team used a focused-ion beam microfabrication tool to modify these specialized cantilevers that are simultaneously very small (9 µm long) but do not ring. However, modified cantilevers were initially too curved for use. The team then developed an innovative and efficient way to straighten out these diving board-like structures, thereby dramatically enhancing the yield of the fabrication process.
The new AFM system is optimized for single-molecule force spectroscopy, a technique used to mechanically measure the folding and unfolding of proteins. The system is capable of detecting changes in a protein’s configuration during the folding or unfolding process because it has an amazing resolution in time of less than 1 µs. The researchers responsible for this feat of nanoengineering included research associates Devin Edwards and Robert Walder, undergraduate students Jaevyn Faulk and Matthew Bull (now a graduate student at Stanford), Aric Sanders of NIST, Marc-Andre LeBlanc and Prof. Marcelo Sousa of the University of Colorado Boulder, and Fellow Tom Perkins.
The researchers used their new custom AFM system to monitor the unfolding of a single polyprotein consisting of 59 amino acid subunits. They found they were able measure the process with 50–100 times better time resolution than traditional AFM-based force spectroscopy and see small changes better than ever before. The new capability bodes well for future work in the Perkins Lab.
“We want to watch biological processes like protein folding on ever faster time scales,” Perkins said. “Now we can do this more easily (than before) with a lot more precision. We are at the point where we can actually watch proteins fold and unfold with microsecond time resolution.”
The researchers proved that their new cantilevers work well on a commercial AFM, which can process more samples and work faster than a custom system. The commercial AFM was retrofitted with a special small laser detection system with a tiny 3-µm circular spot size that just fit on the tiny reflective gold surface on the top of the cantilever. Using this system on a commercial AFM opens the door to other labs adopting the new cantilever technology for biological assays, potentially leading to better understanding of the complex roles protein folding plays in various diseases, and perhaps leading to new drug treatments.––Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.