Researchers in the Kapteyn/Murnane group have decided to use soft X-ray bursts to watch the interplay of electronic and atomic motions inside a molecule. Such information determines how chemical bonds are formed or broken during chemical reactions.
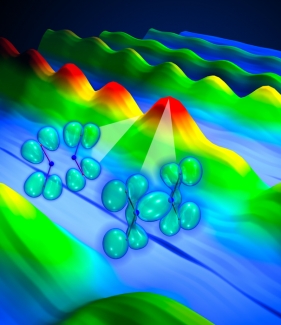
A recent study using this technique was featured on the November 21, 2008, cover of Science magazine. In it, research associate Wen Li joined forces with graduate students Xibin Zhou and Robynne Lock, Fellows Henry Kapteyn and Margaret Murnane, and their theorist colleagues from Canada’s National Research Council. The scientists first used a short burst of laser light to gently "kick" a molecule of dinitrogen tetroxide (N2O4). This molecule is composed of two V-shaped nitrogen dioxide (NO2) subunits linked via a floppy shared-electron bond between the two N atoms.
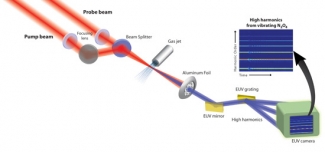
Li wanted to probe how electron energy levels in a molecule (and the shape of their electron clouds) change as the N-N bonds are stretched and compressed like molecular slinkies. To do this, he used a second short burst of laser light to pluck an electron out of each vibrating molecule. The laser field then accelerated the free electrons away from the molecules, then back toward them like a boomerang. In less than 2 fs, the electrons smashed back into the same molecule they came out of. This recombination released energy as an X-ray burst.
Li and his colleagues used the emitted X-rays from the vibrating N2O4 molecules to see how the electronic energy levels changed as the shape of the molecule changed. The scientists found that when the N-N bonds were extended, a bright burst of X-rays was emitted. A burst of X-rays can only happen when a plucked electron recombines with the ion and liberates its excess kinetic energy in the form of X-rays. In this case, the electron plucked from the molecule had an easy time recombining back into an electron cloud (orbital) of the ion that was similar to the one it came from. This orbital is shown on the right in the figure above.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.