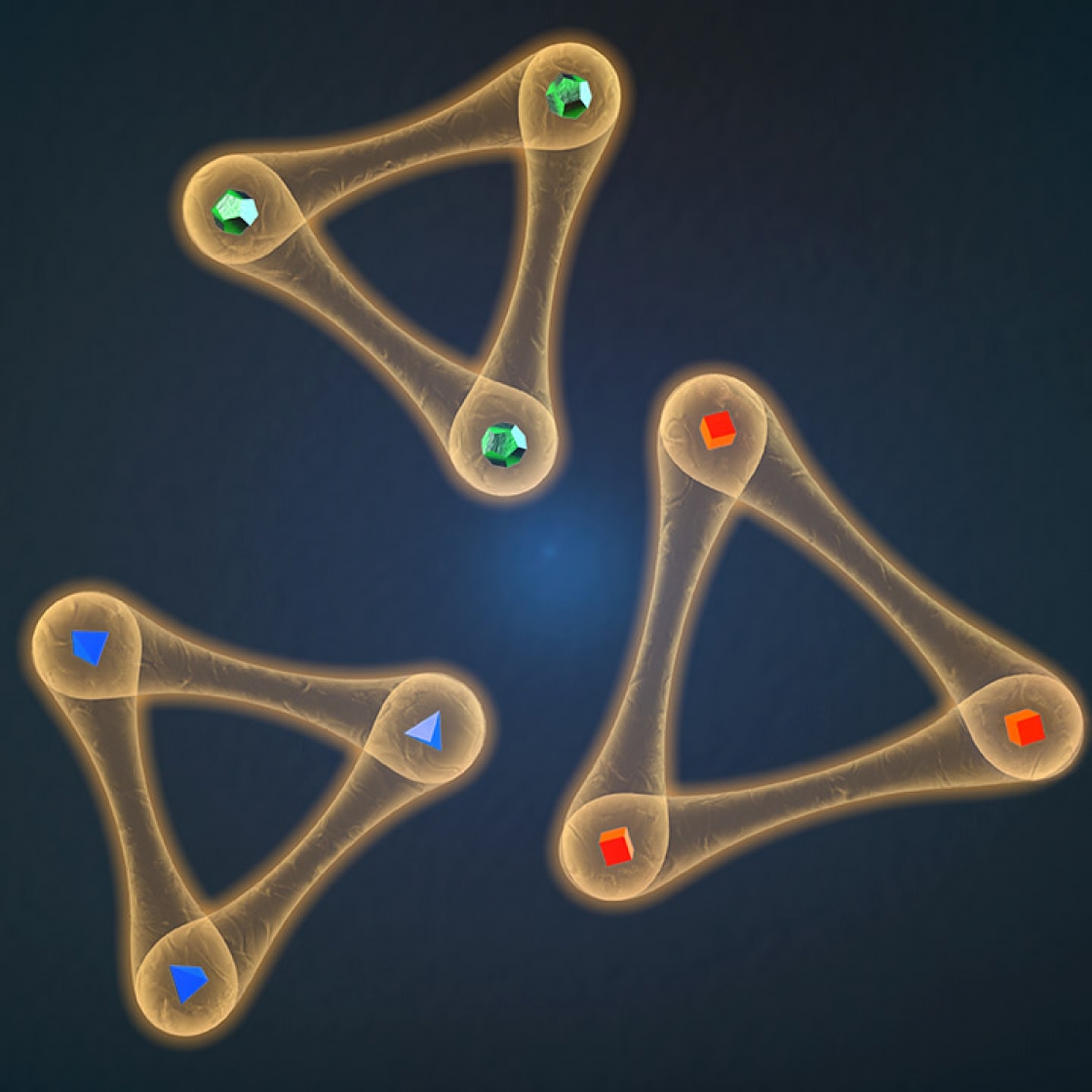
We understand pretty well how a single atom behaves. Two atoms interacting with each other? Still solvable. But it becomes exponentially more complicated to characterize how three atoms or particles interact with each other, explained Xin Xie, a graduate student in the Cornell Group at JILA.
“We study three-body physics because there are still mysteries in this interaction,” Xie said.
Those interactions—whether particles will repel each other, smash together or just orbit each other in perfect harmony—dominate the quantum world. Understanding how those forces work inside a simple hydrogen atom, with its single positive proton and negative electron, is relatively easy, explained JILA Fellow Eric Cornell. But most atoms are much more complicated.
“Atoms aren’t like protons. They’re full of pulleys and bells and whistles,” he said. All of those structures in the “guts” of each species of atom meant that when three atoms got too close to each other, no mathematical formula could predict how all three would interact, Cornell said.
But years of experimental data found there was a sweet spot, a universal range in which the behaviors of three atoms can be decomposed into the more solvable two atom problem. At that range, the atoms are stopped en route to each other, keeping them at just the right distance.
That range was dictated by the van der Waals force. And by knowing the strength of the van der Waals force between two atoms, we can easily predict the shortest distance that three atoms can get without either smashing into each other or repelling off each other. It didn’t seem to matter which species of atoms you looked at; the van der Waals force always dictates three-atom interactions.
For the last decade, this van der Waals universality had been pretty widely accepted…until now. Xie and the Cornell Group recently found this universality has a limit. Their findings raise an important question: When it comes to three-body interactions, just how important are those pulleys, bells and whistles, those innate structures that make up an individual species of atom?
“A long time ago people thought these structures were so important. Then people found that maybe they’re not that important,” Xie explained. “But then we claim that they still matter…it can cause some deviation from the van der Waals universality.”
Take it to the limit
To find the limit, you have to really dig down and look at the atoms very closely, Cornell said. When they’re warm, atoms in a gas cloud bounce around like billiard balls. To measure their interactions, you need to slow the atoms down, and that means making them cold—really cold.
Xie and her team used lasers to bring a cloud of potassium atoms down to 300 nanoKelvin, about -459 degrees Fahrenheit, hovering just above absolute zero.
Then they change the magnetic field around the atoms to force them to interact. As the atoms interact, the cloud decays. The more strongly the atoms interact, the faster it decays. The decay rate reveals information on the spatial extent of a three-atom system.
But Xie and her team found their potassium atoms does not quite fall into the universal group, and within a very narrow margin of error. Clearly, van der Waals universality was not as universal as it seemed.
“Sometimes, those ‘guts’ of the atom matter,” Cornell concluded.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.