The Kapteyn/Murnane group has measured how long it takes an electron born into an excited state inside a piece of nickel to escape from its birthplace. The electron’s escape is related to the structure of the metal. The escape is the fastest material process that has been measured before in the laboratory––on a time scale of a few hundred attoseconds, or 10-18 s. This groundbreaking experiment was reported online in Scienceon June 2, 2016. Also in Science on July 1, 2016, Uwe Bovensiepen and Manuel Ligges offered important insights into the unusual significance of this work.
To document the electron’s great escape from nickel, the group used a high-harmonic generation-based measurement scheme that promises to open the door to measurements of many processes that occur inside atoms, molecules, liquids, and solids. These processes occur too fast to be measured with ordinary techniques in the laboratory.
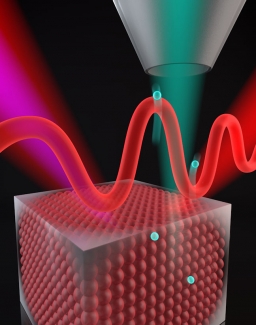
To make the electron-escape measurement, the researchers aimed a sequence of high harmonics of femtosecond laser light at a sample of nickel. The different colors (i.e., photon energies) of the harmonics kicked electrons out from different energy levels in the crystal structure of the nickel, sending them towards the surface. Some electrons escape almost instantaneously. But other electrons––if their energy coincides with a high-energy nickel state––are temporarily grabbed by the high-energy state and linger there for a few hundred attoseconds.
The challenge was how to measure this linger time, or lifetime. Here the researchers took advantage of the weirdness of quantum mechanics. The researchers shined a laser beam onto the sample at the same time as the high harmonics, making it possible for the electrons to absorb different numbers of harmonics and laser photons and still end up in the same final state. This process gave rise to interferences between the different quantum paths taken by the electrons. The researchers analyzed how these quantum interferences changed as the time delay between the laser and high harmonic pulses was changed. The analysis showed that it was possible to determine exactly how long the high-energy nickel band temporarily grabbed onto some electrons before they finally were able to escape for good!
This study added new insights to our understanding of how light ejects electrons from solids in a process called the photoelectric effect. Historically, explaining the photoelectric effect played a major role in the development of quantum mechanics. Over a century later, the group has captured the photoelectric effect in real time to study how electrons bounce off each other (a process called scattering) or rearrange to repel electric fields from a metal (a process called screening). Because electrons are so small and move so quickly, both screening and scattering occur in tens to hundreds of attoseconds. This study demonstrated that scientists are able to directly investigate both processes, which formerly were very challenging to understand and probe.
The researchers responsible for uncovering this great escape include research associate Zhensheng Tao, graduate student Cong Chen, Fellows Henry Kapteyn and Margaret Murnane, and colleagues from NIST and the University of Wisconsin-Madison.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.