When Andy Almand-Hunter and his colleagues in the Cundiff group shined a laser on a sample of gallium arsenide (GaAs), the last thing they were expecting to create was a fog of liquid-like quantum droplets, which the group named "dropletons." Dropletons are a new, stable form of matter much like an ordinary liquid—with one key difference.
Unlike normal everyday liquids, quantum droplets contain charged particles. The particles are negatively charged electrons and positively charged “holes.” Holes are like bubbles created when electrons in GaAs are excited by light.
When it discovered the quantum droplets, the Cundiff group was actually investigating the use of intense laser light to generate biexcitons, which are molecule-like structures in GaAs made of two excitons. Excitons are hydrogen-like quasi particles made of an electron and a hole.
“But the experiment didn’t behave at all in the way we expected,” Almand-Hunter said. “We expected to see the energy of the biexcitons increase as the laser generated more electrons and holes. But, what we saw when we did the experiment was that the energy actually decreased!”
The energy decrease meant that the researchers certainly were seeing something other than biexcitons. In fact, they weren’t sure what they had made. At this point, experimentalists Hunter, former research associate Hebin Li, and Fellow Steve Cundiff consulted their theorist colleagues at Philipps-University Marburg in Germany.
The German collaborators came up with the idea that the experimentalists had made quantum droplets. A quantum droplet is a structure containing multiple electrons and holes (for example, 4, 5, or 6 of each) that is in between that of a traditional atom with positively charged nucleus surrounded by negatively charged electron(s) and an older model of the atom that viewed it as a positively charged sphere with electrons embedded in it. The droplets behaved quantum mechanically because they contain only a few electrons and holes.
Dropletons aren’t made up of multiple excitons because the electrons and holes in them are not bound into pairs. In a quantum droplet, all of the electrons interact equally with all of holes and vice-versa. In an excitonic molecule, such as a biexciton, the electrons and holes form excitons, which then form molecules. In this case, each electron primarily interacts with a single hole. It’s completely different with quantum droplets.
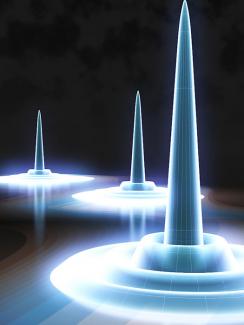
According to the quantum-droplet theory calculations (as represented in the figure), the electron is in the middle of the tall structure in the middle of the dropleton. The hole and the electron are most likely right on top of each other. However, the hole could also be just above, just below, or even next to the electron. The next most likely location of the hole is somewhere on the first ring. The third most likely location for the hole is on the second ring. The least likely location is in the gaps between the rings. As the density of electrons and holes increases inside a droplet, so too does the number of rings, as shown in the background of the figure.
The experimental observations made by Almand-Hunter and his colleagues fit perfectly with the new theory. The researchers realized that they had inadvertently created a quantum fog of electrons and holes in close proximity to one another. In the process, they had discovered a new quasiparticle as stable as an atom or a solar system. This story is featured on the cover of the February 27 online issue of Nature.---Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.