According to the laws of quantum mechanics, identical fermions at very low temperatures can’t collide. These unfriendly subatomic particles, atoms, or molecules simply will not share the same piece of real estate with an identical twin. A few years back, researchers in the Ye lab considered this unneighborly behavior a big advantage in designing a new optical atomic clock based on an ensemble of identical 87Sr atoms. They (along with every other physicist in the world) assumed that using fermions meant they wouldn’t have to worry about atomic collisions causing frequency shifts when these atoms were cooled to sufficiently low temperatures.
Of course, things are never as simple as you expect them to be. In 2008, the Ye group’s optical atomic clock team identified tiny frequency shifts in their clock caused by — colliding fermions. In response, Andrew Ludlow, the graduate student who was leading the project, did the smart thing and graduated. The job of figuring out why some 87Sr atoms were colliding fell to research associate Gretchen Campbell, graduate students Mike Martin, Sebastian Blatt and Travis Nicholson, research associate Matt Swallows, Fellow Jun Ye, and their colleagues from NIST (now including Ludlow). Former JILAn Marty Boyd and Visiting Fellow Jan Thomsen also helped out.
The good news: the researchers found no violations of the laws of quantum mechanics. The interaction of their laser-based precision measurement technique with the optical lattice confining the 87Sr atoms was responsible for the frequency shifts.
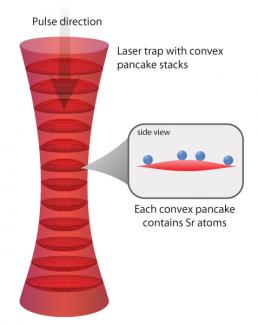
Here’s what Campbell and her colleagues discovered. Their frequency measurements required focusing a laser beam on a 87Sr atom-filled lattice. However, because the lattice is actually slightly curved, the atoms moving around inside it transitioned to their excited states at slightly different rates. During the transition from their ground to excited states, the atoms evolved differently. And, fermions in different superpositions of their ground and excited states are no longer identical. Pairs of atoms that can be distinguished from one another can (and do) collide.
Once they understood what was happening, the researchers were able to devise strategies to reduce (though not entirely eliminate) the number of atom-atom collisions and the resulting frequency shifts. For instance, by lowering the temperature of the clock system during a measurement, they were able to reduce the number of resulting motional states. Fewer motional states helped make the journey from the ground to excited states for different atoms more uniform. The researchers also found that if they excited the atoms to nearly equal superpositions of their ground and excited states, the effects of many atom-atom collisions would average to zero. Using both techniques, the researchers were able to significantly reduce the uncertainty caused by the frequency shifts, enhancing the performance of what was already the world’s best neutral atom-based optical clock. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.