The new molecules are as big as a virus. They’re ultracold. And, they’re held together by a ghostly quantum mechanical force field with the energy of about 100 billionths of an electron volt.
These strange diatomic rubidium (Rb) molecules are the world’s first long-range Rydberg molecules. They were recently formed in Tilman Pfau’s laboratory at the University of Stuttgart from an ultracold cloud of Rb atoms. One neat aspect of the new experiment is that in 2000, Fellow Chris Greene’s collaboration with Hossein Sadeghpour (ITAMP/Harvard) and Alan Dickinson (Newcastle) predicted the novel chemical bonding process that would give rise to Rydberg molecules in an ultracold gas.
To make a Rydberg molecule, you need a Rydberg atom — an atom in an excited state in which at least one electron roams far from its parent nucleus (in an elongated elliptical orbit). Lasers are a good tool for making some Rydberg atoms inside a cloud of ground-state Rb atoms.
Next comes the exciting part. If a roaming Rydberg electron moves close enough to an ordinary ground-state atom, the electron can capture and hold onto the ordinary atom, forming a Rydberg molecule. The electron captures the ordinary atom when the latter wanders inside its orbit — which can occur at interatomic distances of 100 nm or more! The Rydberg electron then binds the molecule much like a sheep dog keeps its flock together by running around the perimeter of its territory and continually nudging strays back toward the center.
In other words, once an energetic Rydberg pup captures a ground-state atom, it races around its orbit keeping the second atom from drifting away. The molecule will stay bound for about 18 µs if the environment around it remains ultracold. Warm things up just a tiny bit, and the weakly bound Rydberg molecule falls apart — which is why there aren’t any already hanging out in out-of-the-way places in the Universe.
The fact that Rydberg molecules can’t exist outside the laboratory is probably why extreme "sheep" herding hasn’t been added before now to the familiar roster of chemical bonds that includes ionic, covalent, hydrogen, and van der Waals bonds. Thanks to the Stuttgart experiment, though, it will be there from now on.
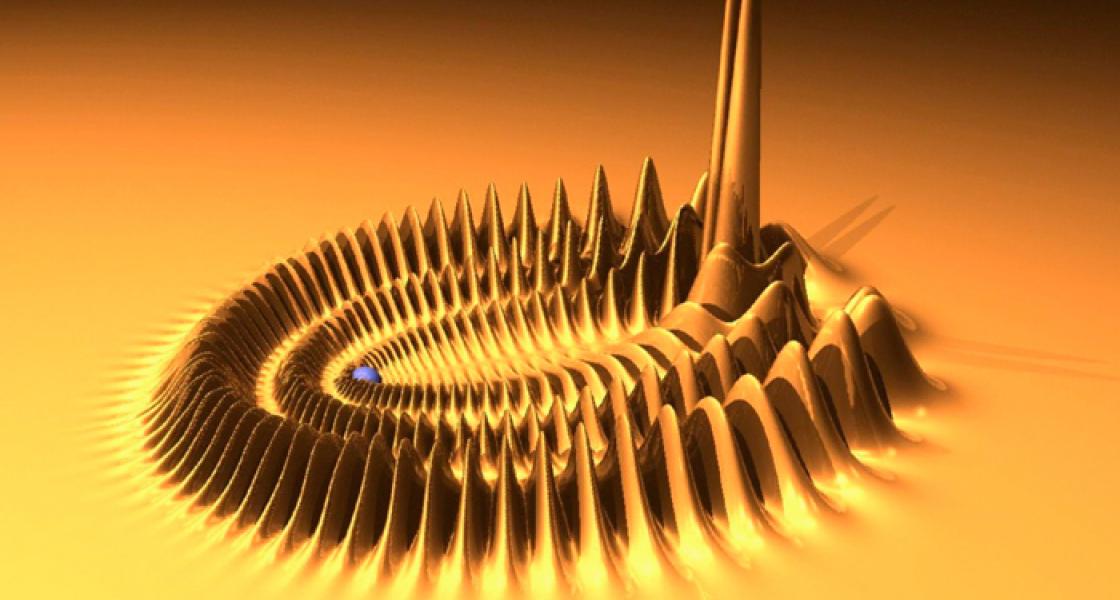
The fact that diatomic Rydberg molecules can be created in the laboratory opens up some exciting possibilities. Greene would like to see the creation of more strongly bound diatomic Rydberg molecules — by further exciting the molecules minted at Stuttgart. He and his collaborators have already predicted the electronic wave functions of two such molecules, one of which resembles a butterfly. The other, shown here, looks a lot like the trilobites that ruled the ancient seas. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.