The Kapteyn-Murnane group has come up with a novel way to use fast bursts of extreme ultraviolet light to capture how strongly electrons interact with each other in materials. This research is important for figuring out how quickly materials can change their state from insulating to conducting, or from magnetic to nonmagnetic. In the future such fast switching may lead to faster and more efficient nanoelectronics.
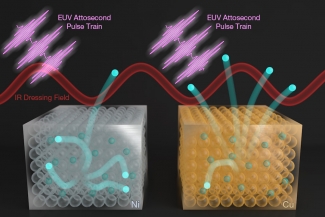
In this work, graduate student Cong Chen, research associate Zhensheng Tao, and their colleagues used sequences of attosecond (10-18 s) bursts of extreme ultraviolet light to compare how long it took an electron to be emitted from the same energy levels, or bands, in two different metals––copper (Cu) and nickel (Ni).
In the past, scientists thought that the photoelectric effect, in which a high-energy photon kicks out an electron from a material, was instantaneous. More recently, with the advent of shorter and shorter laser pulses, scientists have been able to investigate such super-fast processes directly. And, they have discovered many surprises. In Chen’s and Tao’s work, the researchers found that the photoelectron lifetime was 100 attoseconds longer in Cu than that in Ni––even though the two metals sit side by side in the periodic table and the photoelectrons were ejected from the same bands.
There is a key difference between the two materials, however. Nickel has more high-energy states that are normally empty. Thus, when an electron is kicked out of nickel, it has a significant probability of interacting with another electron on its way out. This interaction sends the photoelectron into one of nickel’s empty states, and the photoelectron loses energy in the process. If this electron-electron scattering process occurs, it is difficult to measure the photoelectron. The result is a reduction of the photoelectron lifetime in nickel.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.