When it comes to working with atoms, or other quantum systems, some physicists prefer to keep them cold. That generally works well, but Earth is a warm place.
“We live in a heat bath,” joked Molly May, a JILA graduate student in the Raschke group.
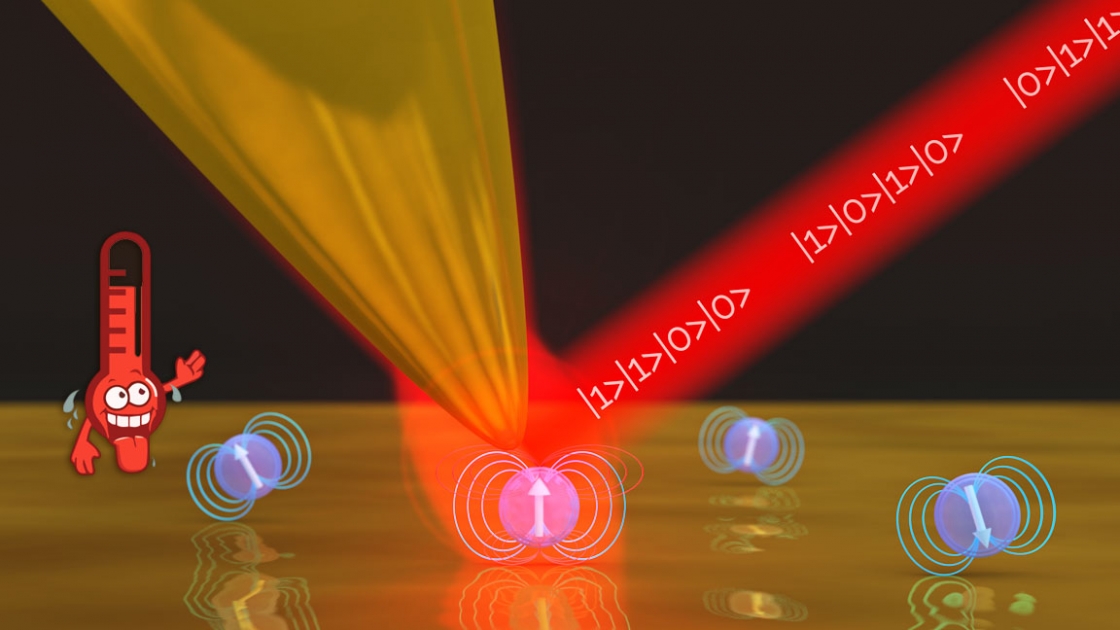
Despite their small size, atoms have the ability to contain and carry information which makes them a promising platform for quantum sensing, metrology, and information processing. However, artificial atoms in the form of quantum dots can also encode information, and the information in those quantum dots can be controlled with light, May explained, making them attractive for these new applications.
But that gets tricky. Quantum dots and atoms tend to lose the information rapidly to their surroundings, so one has to work quickly and precisely. That typically means working in extremely cold temperatures. Extremely cold temperatures isolate the atoms, preventing them from interacting with their environment—which makes them easier to work with and control.
And if controlling and working with atoms is restricted to cold temperatures, that makes it harder to take advantage of atoms’ potential applications outside the lab, added JILA Fellow Markus Raschke. Now an advance from the Raschke group at JILA and their collaborators at the University of Maryland has brought quantum dots out of the cold, allowing them to work at room temperatures with precision and control.
Writing on a nanoscale
The Raschke group works with quantum dots that are about 8 nanometers across, more than 1000 times smaller than a human hair, which is big compared to atoms.
Writing information on a quantum dot involves changing its energy level from its ground state to an excited state. And photons—tiny packets of energy that make up light—can do that when they have a strong interaction (or are coupled) with the dot or atom.
But compared to the quantum dots or atoms, light—especially visible light—has a very long wavelength, almost a hundred times larger than the quantum dot. As a result, it has a very weak interaction with the quantum dot. Light needs to shine on the quantum dot for a long time to excite its energy level–i.e. write information on it.
However, over that time, the movement and coupling of the atom knocks its excitation out of phase, hitting other atoms and molecules, or simply internal processes. That phase decoherence causes the atom to lose its information to the surrounding environment.
There are two ways to work around this. One option is to work in high vacuum and at ultra-cold temperatures, usually around a few micro-Kelvin, to reduce the chances of this phase decoherent interaction. That requires complex cooling or refrigeration techniques in a lab, which isn’t always ideal.
The other option is to squeeze your light into a space smaller than its wavelength, ideally comparable to the size of the quantum dot. Using nanoscale antennas, e.g., made by metallic nanostructures, you can create an optical nano-cavity, squeezing the light into a smaller space. But those cavities are static. You either hit the dot or you don’t, May explained.
“Even when you get lucky and find a quantum dot, you cannot tune the cavity or control the interaction,” she added.


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.