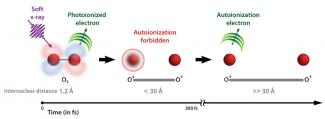
An oxygen molecule (O2) doesn't fall apart so easily — even when an X-ray knocks out one of its electrons and superexcites the molecule during a process called photoionization. In this process, the X-ray first removes an electron from deep inside the molecule, leaving a hole in O2+. Then, an outer electron can fall into the hole, and a second outer electron gets ejected, carrying away any excess energy. The loss of the second electron is known as autoionization, or Auger decay.
Surprisingly, in superexcited O2+, the second electron can take a really long time to be ejected — up to 300 fs. During this time, the pieces of the oxygen molecule, consisting of an excited O atom and a positively charged O ion, move at least 25 times farther apart than in a normal O2 molecule before the second electron is ejected. When the second electron is ejected, the molecule falls apart. The basic sequence of events is shown in the figure.
So what's happening inside superexcited O2 molecules that keeps them intact for so long? A very complicated process, according to former JILAns Etienne Gagnon, Arvinder Sandu, and Robin Santra; research associate Wen Li, Fellows Margaret Murnane and Henry Kapteyn; and their colleagues from Kansas State University.
The scientists recently monitored the breakup of molecular oxygen after zapping the molecule with a fast burst of soft X-rays. They found that the molecule itself suppressed ejection of the second electron until the fragments of the molecule were separated by 30 Å (3 nm) or more. Essentially, the second electron felt an attraction to each of the O fragments that kept it bound between the two ions until they were so far apart, they hardly interacted at all.
Even after this separation, the researchers found that the electron did not immediately leave the atom. Rather, it transformed into a "Feshbach resonance" state in which the electron remained bound, albeit with a negative binding energy. (Actual ionization required a change in the internal state of the atom.) The existence of negative-binding energy states was completely unexpected because these states represent a new “superheated” phase of atomic matter.
To see the exotic Feshbach resonance atoms, the group used an ultrafast laser to induce the atom to ionize and then observed the fragments using sophisticated detectors that allowed them to see the electron and ions in coincidence. This detection strategy made it possible to study the break-up events one at a time. It also allowed the researchers to test computer models and our basic understanding of the structure of atoms excited far from their ground state. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.