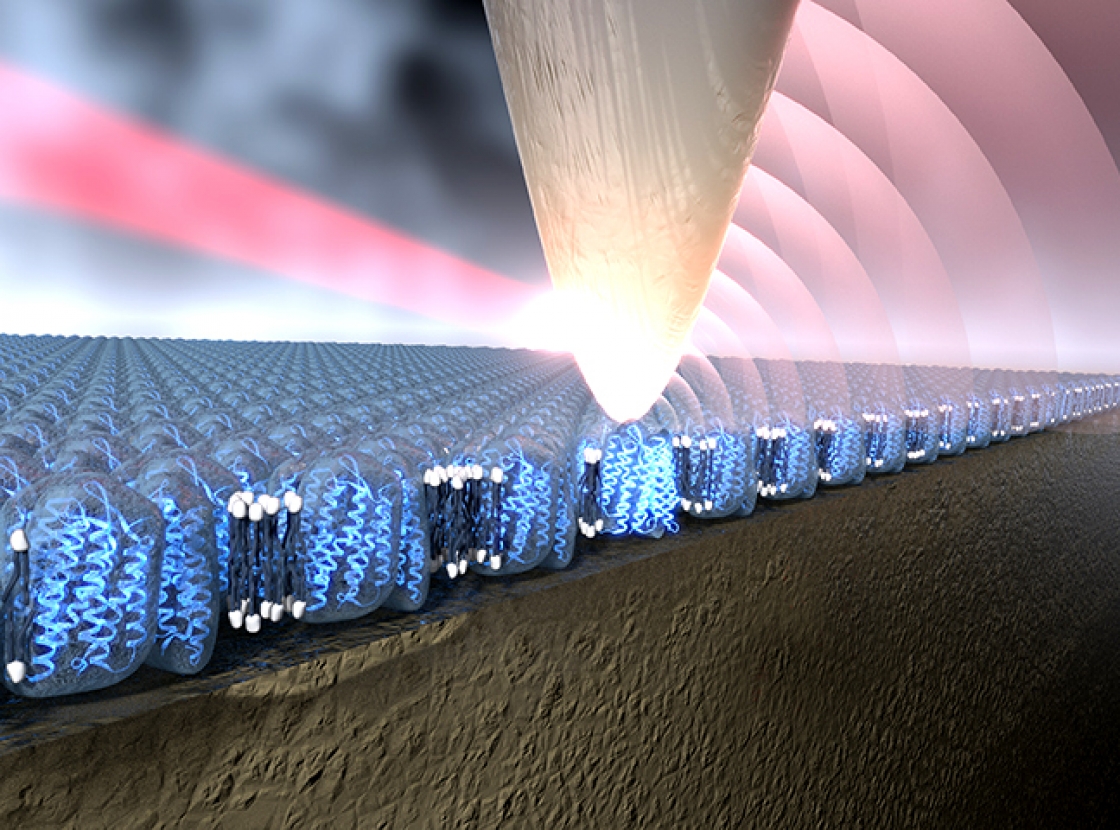
The groups of Fellow Adjoint Markus Raschke and Fellow Tom Perkins joined forces recently to shine light onto a bacterial membrane protein called bacteriorhodopsin (bR). They used a new infrared (IR) light imaging system with a spatial resolution and chemical sensitivity of just a few bR molecules. In their experiment, the tip of an atomic force microscope (AFM) acted like an antenna for the IR light, focusing it onto the sample. The AFM-tip antenna then helped capture the signal emitted by the bR protein and send it back to a detector for identification and location. The AFM-tip antenna worked a lot like a cell phone antenna except that it talked to protein molecules.
The protein cell phone is actually an IR nano microscope called s-SNOM (scattering scanning near field optical microscope). The new work has opened up imaging of biological and chemical structures 5000-fold smaller than the diameter of a human hair. In particular, s-SNOM provides a label-free method to probe the chemical composition of a material, easily distinguishing proteins from other chemical constituents such as the fat molecules making up a membrane. Until now, such chemical distinctions were impossible to “see” with ordinary light microscopy and too large and complex to analyze with x-ray crystallography.
With the new method, the researchers were able to identify the bR protein with a spatial resolution of 20 nm, or the length of 2–3 bR molecules. Plus, the researchers were able to acquire IR spectra of just a handful of protein molecules, compared to about 10,000 molecules required for getting good spectra with a regular IR microscope.
They accomplished this feat by first using a quantum cascade laser to excite amide groups in the protein. Amide groups, which contain carbon, oxygen and nitrogen, vibrate like crazy when excited by IR light. Then, with the help of the AFM-tip antenna in the s-SNOM setup, the researchers tickled the proteins, causing them to reemit the IR light. That made it possible for them to zero in and “see” the bR protein backbones. The researchers responsible for this feat included Raschke, Perkins, research associate Sam Berweger, former undergraduate student assistant Duc Nguyen, research associate Eric Muller, and their colleague Hans Bechtel of Lawrence Berkeley National Laboratory.
The Raschke group is already working on improving the new imaging method with the goal of resolving single molecules, including some in liquid. For its part, the Perkins group plans to continue its quest to better understand membrane proteins, which are the target of 50% of all current and future drugs. The two groups plan to continue their collaboration with the goal of one day being able to probe and understand the structures and functions of the myriad of constituents of living cells. And, they want to do all this in real time and under realistic conditions for life.—Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.