RNA molecules can perform amazing biological feats, including storing, transporting, and reading genetic blueprints as well as catalyzing chemical reactions inside living cells. To manage the latter feat, RNA molecules must rapidly fold into an exact three-dimensional (3D) shape. Understanding how RNA accomplishes this is a major scientific challenge. Former JILA postdoc Jose Hodak, Christopher Downey (doctoral candidate in Chemistry and Biochemistry), JILA graduate student Julie Fiore, Chemistry and Biochemistry Professor Arthur Pardi and Fellow David Nesbitt are meeting this challenge head on.
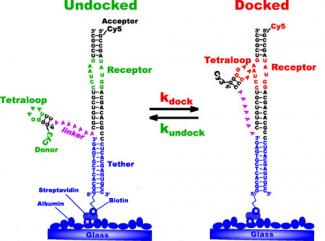
The researchers tether small pieces of a large RNA molecule that contain one site critical to proper molecule folding to a glass cover slip. As shown in the figure of this RNA construct on the right, the critical site, called a tetraloop, can bond lightly, or dock, to a receptor region as part of the folding process. As it turns out, regions like this are what form and maintain the 3D structure of large RNAs. They are, in essence, molecular Velcro.
Nesbitt and his colleagues want to understand what makes molecular Velcro stickier as a means of understanding RNA folding. To do this, they study the process of docking and undocking by strategically labeling the tetraloop and its receptor with different dyes such that when the tetraloop is undocked, the RNA strand emits mostly green light when illuminated by a laser. When the tetraloop is docked to the receptor, the RNA strand emits mostly red light. With this method (called single-molecule fluorescence resonance energy transfer, or smFRET), the researchers can study the conditions that affect the folding process.
Recently the researchers studied how magnesium affects thousands of RNA constructs tethered to a single cover slip. By observing them one molecule at a time with an ultrasensitive microscope for periods of 10 to 30 seconds, they discovered that the docking rate increased 12-fold with increasing concentrations of magnesium, as shown in the scanned images on the right. They also found that the undocking rate actually slowed slightly as well. In other words, magnesium made it easier for the two halves of the molecular Velcro to find each other and the Velcro was stickier when they did! The researchers also observed that a significant fraction of the RNA constructs docked even in the absence of magnesium—the first time anyone had observed this behavior.
In the future, Nesbitt and his colleagues plan to study other interacting regions of complete RNA molecules with the goal of understanding all the pieces of molecular Velcro that govern the folding of an entire particle. The researchers reported their findings in the July 17, 2005 issue of the Proceedings of the National Academy of Sciences. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.