The molecular recognition of ions in aqueous solution has been a major area of chemical research for decades. Nature’s own molecular recognition apparatus largely relies on proteins, but the importance of ion receptors is not limited to the natural world, and synthetic molecular receptors have long been a focus of attention in supramolecular chemistry. Applications of molecular recognition of ions address the need to measure and control the concentration of ionic species in numerous chemical contexts, ranging from ionophoric antibiotics to sensing, materials science, and separation/extraction science. Ion recognition also has impact on important issues regarding food and water sources, such as the safety of public water supplies, or pollution with nitrate and phosphate from agriculture.
Despite the decades-long work on molecular recognition complexes, a complete, predictive understanding of noncovalent interactions in aqueous host-guest systems has not been achieved to date. Although modern computational approaches have made enormous progress, the agreement between experimental data on the thermodynamics and kinetics of guest-host interactions and quantum chemical or molecular dynamics results still has significant potential for improvement. As a consequence, there is still a need for a detailed, fundamental understanding of ion receptors and binding competitiveness in solution in order to advance the rational design of synthetic hosts for ionic guests. Experimental work in solutions (in particular NMR spectroscopy) has been instrumental for obtaining structural data of the host-guest complexes themselves. However, effects of solvation are much more difficult to account for, particularly the interaction of the immediate solvation environment (i.e., the first solvation shell) with both receptor and ion, and its effect on the structure of and intermolecular forces in host-guest complexes. For aqueous environments, solvation effects have frequently been described in the framework of the hydrophobic effect and the Hofmeister effect, which are usually discussed in terms of averaged water-solute interactions that depend on solute shape and size.
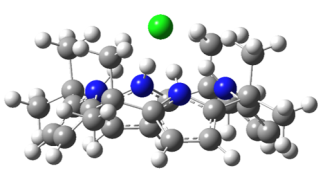
In this project, we aim to develop a detailed, molecular-level understanding of the non-covalent interactions in molecular recognition for selected prototypical ion receptors. We will do so by employing electrospray ionization to bring molecular receptor complexes from solution into the gas phase, such as [Cl-·(omC[4]P)]. We then use cryogenic ion spectroscopy to obtain vibrational and/or electronic spectra for our target complexes. We interpret our spectra with the help of quantum chemistry calculations. The expected outcome of these experiments and of the accompanying computational analysis is information on the structure and intermolecular forces of selected ionic host-guest system. This information will be highly useful for the evaluation of current and future computational treatment for non-covalent interactions in molecular recognition. We expect that this knowledge will contribute to the predictive design of future ion receptors in molecular recognition applications.
You can read about our work here, here, and here.
 This work is funded through an award from the NSF Division of Chemistry.
This work is funded through an award from the NSF Division of Chemistry. 


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.