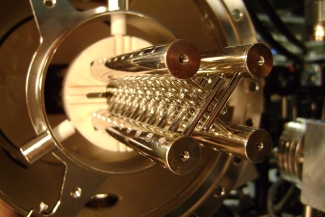
Pulsed deceleration utilizes pairs of oppositely charged rods to create the longitudinal potential hill for cold polar molecules to climb. Before they can fall down the potential hill and regain their kinetic energy, the pin pairs are quickly discharged. This process is repeated with successive stages of electrodes until the molecules have been sufficiently slowed to be loaded into an electrostatic trap. Additionally, transverse guidance of the molecules is achieved because the molecules are attracted to the minimum of electric field along the center of the decelerator. Successive pin pairs are orientated orthogonally to one another, to guide the molecules equally in both transverse dimensions.
Current research involves the trapping of slowed hydroxyl radicals (OH) for cold collision studies with rubidium (Rb). For more information, visit our cold molecular collisions page.
In the past, we trapped slowed ammonia molecules (ND3) to study density fluctuations and trap loading dynamics. We used Monte Carlo simulations to optimize the trap loading timing. An example of loading the electric trap can be seen in the following phase-space and coordinate-space simulations:


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.